Abstract
The aim of this study was to investigate the role of miR-29b in the regulation of decidua-derived mesenchymal stem cells (dMSCs) to influence the pathogenesis of pre-eclampsia (PE). dMSCs were isolated from decidua tissue and characterized. The expression of miRNAs was evaluated by quantitative PCR. Overexpression and inhibition of miR-29b were achieved in dMSCs, and the effect of miR-29b overexpression on the migration and angiogenic potential of human umbilical vein endothelial cells (HUVEC) was assessed. Furthermore, we performed bioinformatic analyses, luciferase reporter gene assays, and gene expression analyses to identify the potential targets of miR-29b and to verify the effect of target genes on dMSC function. Upregulation of miR-29b was confirmed in severe PE patients. Overexpression of miR-29b attenuated cell proliferation of dMSCs. Overexpression of miR-29b in dMSCs decreased the migratory and tubule formation ability of HUVECs. Histone deacetylase 4 (HDAC4) was identified as a target gene of miR-29b. Decreased migration and tubule formation in HUVECs was observed upon incubation with conditioned medium from dMSCs treated with the HDAC inhibitor trichostatin A. Our results demonstrated that upregulation of miR-29b in dMSCs has an important paracrine effect and might be involved in the development of PE.
Keywords: Pre-eclampsia, mesenchymal stem cells, miR-29b, migration, angiogenesis
Introduction
Pre-eclampsia (PE) is a multisystem disorder characterized by new-onset hypertension and proteinuria during pregnancy. It has been reported that the incidence of PE ranges from 3-8% of all pregnancies [1]. This condition is a major cause of maternal mortality and morbidity, preterm birth, perinatal death, and intrauterine growth restriction [2]. During the past decade, extensive research addressing this disorder has been performed; however, the pathological mechanisms that lead to PE are still poorly understood.
Mesenchymal stem cells (MSCs) represent a heterogeneous subset of pluripotent progenitor cells that can be derived from the in vitro culture of various adult tissues and organs (such as bone marrow, fat, umbilical cord, and placenta) [3]. MSCs also have the capacity of differentiate into multiple lineages of mesenchymal tissues including adipose tissue, bone, cartilage, and muscle; in this regard, MSCs might be involved in the maintenance of various microenvironments in vivo [4]. In recent years, there has been considerable interest regarding the role of MSCs in the treatment of PE. Most recently, Liu et al isolated MSCs from decidua tissue and proved that these decidua-derived MSCs (dMSCs) could ameliorate T helper cell-induced PE like symptoms via suppressing the expression of tumor necrosis factor α (TNF-α) [5]. However, the in situ effect of dMSCs in the pathogenesis of PE has not been fully elucidated.
miRNAs, a class of endogenous highly conserved noncoding small RNAs, exert their suppressive effect on post transcriptional gene expression via target gene degradation and translational inhibition [6]. Growing evidence has proven that miRNAs participate in various biological processes [7,8], and recent studies suggested that miRNAs are involved in the pathogenesis of PE [9,10]. Most recently, Wang et al found upregulation of miR-29b in severe PE (sPE) patients and showed that this molecule participates in the regulation of dMSC function [11]. However, the role of miRNA in dMSCs has not been fully explored.
In this study, we identified the role of miR-29b in the regulation of dMSC function and its involvement in the pathogenesis of PE. Our results showed that upregulation of miR-29 in dMSCs had a paracrine effect and regulated the angiogenic potential of human umbilical vein endothelial cells (HUVECs).
Materials and methods
Subjects
Human decidual tissue from sPE patients and control subjects was collected and sterilized at the time of cesarean section delivery at The Second Hospital of Shandong University. The demographic data of the patients are listed in Table 1. The study protocol was approved by the Institutional Review Board of The Second Hospital of Shandong University and was in full compliance with the Declaration of Helsinki. Written informed consent was obtained from each participating individual. The inclusion criteria for sPE was defined as either severe hypertension with maternal blood pressure > 160/110 mmHg, assessed by two separate readings at least 6 h apart, or severe proteinuria at a concentration of > 2 g protein over a 24 h period. Patients with pregnancy complications such as twins, fetal structural or genetic anomalies, or the presence of maternal chronic hypertension, hemolysis, elevated liver enzymes, HELLP syndrome, cardiovascular disease, renal disease, hepatic disease, diabetes, or other immune system disorders were excluded.
Table 1.
Patient characteristics
| Parameter | PE (n = 15) | Control (n = 15) | P value |
|---|---|---|---|
| Age (years) | 27.9 ± 1.3 | 28.3 ± 1.1 | > 0.05 |
| Gestational age at delivery (week) | 37.2 ± 0.2 | 38.4 ± 0.4 | > 0.05 |
| Primiparae (n) | 6 (40%) | 7 (46.7%) | > 0.05 |
| BMI (kg/m2) | 29.1 ± 0.9 | 27.6 ± 1.2 | > 0.05 |
| SBP (mmHg) | 162.5 ± 4.3 | 118.9 ± 4.1 | < 0.05 |
| DBP (mmHg) | 115.3 ± 2.4 | 82.3 ± 3.5 | < 0.05 |
| Proteinuria (g/24 h) | 2.2 ± 0.02 | 0 | < 0.05 |
| ALT (units/l) | 33.7 ± 7.9 | 30.7 ± 6.3 | > 0.05 |
| BUN (mmol/l) | 4.0 ± 0.3 | 3.7 ± 0.2 | > 0.05 |
| Platelet counting (109/ml) | 156.8 ± 21.7 | 191.8 ± 30.8 | > 0.05 |
| Birth weight (g) | 2834.6 ± 174.0 | 3416.3 ± 159.0 | > 0.05 |
| Placenta weight (g) | 484.8 ± 26.5 | 525.3 ± 28.5 | > 0.05 |
BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; ALT: alanine aminotransferase; BUN: blood urea nitrogen; PE: pre-eclampsia.
dMSC isolation
Decidua tissues (collected as outlined previously) were washed with phosphate-buffered saline, cut into small pieces (1-2 mm3) and digested with type IV collagenase at a concentration of 0.25 mg/ml (Sigma, MO, USA) for 2 h at 37°C. The tissues were then filtered through a 100-mm strainer and cells were collected by centrifugation at 200 × g for 5 min. After discarding the supernatant, the cell pellet was resuspended in low glucose Dulbecco’s modified Eagle’s medium (DMEM-LG, Gibco, NY, USA) containing 20% fetal bovine serum, seeded in 6-well plates, and incubated at 37°C and 5% CO2. The medium was then changed every 3-4 days. When the cells reached 80% confluency, they were digested with 0.25% trypsin containing 0.02% ethylenediaminetetraacetic acid (Life technology, CA, USA) and split at a ratio of 1:5. Cell were characterized using phycoerythrin- or fluorescein isothiocyanate-conjugated monoclonal antibodies against human CD34, CD73, CD90, CD105, and HLA-DR (BD Pharmingen, CA, USA); cells at passage 2-5 were used for the following experiments.
HUVEC culture
HUVECs, obtained from Life technologies company, were grown in Medium 200PRF (Life Technologies, Invitrogen™) with low serum growth supplement (LSGS), and were incubated in a humidified atmosphere with 5% CO2 at 37°C.
Quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR)
Total cellular RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. RNA quantification was performed using a Nanodrop 2000 (Thermo, CA, USA). A High Capacity cDNA Reverse Transcription Kit and a TaqMan MicroRNA Reverse Transcription kit were used for mRNA and miRNA reverse transcription, respectively (Applied Biosystems, Foster City, CA, USA). Quantitative RT-PCR was conducted using a One Step SYBR® PrimeScript™ RT-PCR kit (Takara, Dalian, China) for HDAC4 and GAPDH or using TaqMan microRNA assays for miRNA and U6 (Applied Biosystems, Foster City, CA, USA). Expression of the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene and U6 was assessed simultaneously in all samples as internal controls. Relative gene expression was determined using the 2-ΔΔCT method [12].
MTT cell proliferation
dMSCs (2 × 103 cells) were seeded in 96-well plates in DMEM-LG containing 10% FBS and were transfected with various oligonucleotides of miR-29b including pre-miR-control, pre-miR-29b, anti-miR-control, and anti-miR-29b, after overnight incubation. Cells were treated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; 5 mg/ml) at 24, 48, and 72 h time points, and proliferation was measured using a microplate reader (Molecular Devices, Sunnyvale, CA). Results were reported as percent optical density (OD) value relative to that of the control group.
Pre- and anti-miR transfection and collection of conditioned medium (CM)
To overexpress and knockdown miR-29b, pre-miR and anti-miR (respectively) were transfected into dMSCs using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. CM was collected 36 h after transfection, and the expression of miRNA was confirmed by previously described real time RT-PCR methods. For trichostatin A (TSA), CM was collected 36 h after TSA (0.1 μM, Sigma) treatment.
Migration assay
Migration assays were performed using a transwell system as described previously. HUVECs (2 × 104 cells) were resuspended in serum-free Medium 200 and seeded on the upper chamber. The lower chamber was filled with CM. After incubation at 37°C and 5% CO2 for 24 h, the cells were stained with hematoxylin and the cell number in each well was counted in three randomly selected fields (200× magnification) under a light microscope. All experiments were performed in triplicate.
Tubule formation assay
Matrigel (BD Biosciences, CA, USA) was diluted 1:1 with CM described previously and added to 48-well plates for gelling. HUVECs (5 × 104 cells) were seeded in Matrigel (pre-solidified)-coated 48-well plates, and tubular structures were evaluated under a microscope after 24 h incubation. To quantify the length of newly formed tubes, six random phase contrast photos were taken per well, and the length of each tube was measured using Quantity One Image software.
Western blotting
dMSCs with or without pre-miR-29b transfection were lysed in RIPA buffer, which was followed by high-speed centrifugation and quantification using bicinchoninic acid. Cellular proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes. After blocking, membranes were incubated with histone deacetylase 4 (HDAC4) (Sigma). Actin (Santa Cruz Biotechnology, Santa Cruz, CA) was used as the loading control. Appropriate horseradish peroxidase-conjugated secondary antibodies were applied. The protein bands were detected with SuperSignal Ultra Chemiluminescent Substrate (Pierce, Rockford, IL) using X-ray films (Kodak, Tokyo, Japan).
Luciferase assays
The pMIR-HDAC4-3’UTR plasmid, containing the putative binding site of the HDAC4 3’UTR downstream of the firefly luciferase gene, was generated by cloning and inserting a 400-bp sequence, located on the 3’UTR, downstream of the SpeI and HindIII sites of the pMIR-REPORT Luciferase vector (Ambion, TX, USA). For luciferase activity measurements, dMSCs cells were grown in 24-well plates until they reached 60-70% confluence, which was followed by co-transfection with a Renilla luciferase plasmid (Ambion) as a control for transfection efficiency, along with pre-miR-29b or negative control, using Lipofectamine 2000 (Invitrogen). The firefly and Renilla luciferase activities were assessed after 48 h with the Dual Luciferase Reporter 1000 Assay System (Promega, WI, USA).
Statistical analyses
All statistical analyses were performed using SPSS v18 software (SPSS, Chicago, IL). Data are presented as mean ± SD. A Student’s t-test was used to examine differences between groups. P < 0.05 was considered significant.
Results
Upregulation of miR-29b in sPE dMSCs
Quantitative real-time PCR was employed to evaluate dysregulated miRNAs in sPE patients and control subjects. We found that the expression of miR-29b and miR-30a was upregulated in dMSCs from sPE patients, whereas no significant change was found for the expression of miR-210 and miR-152 in these sPE dMSCs (Figure 1).
Figure 1.
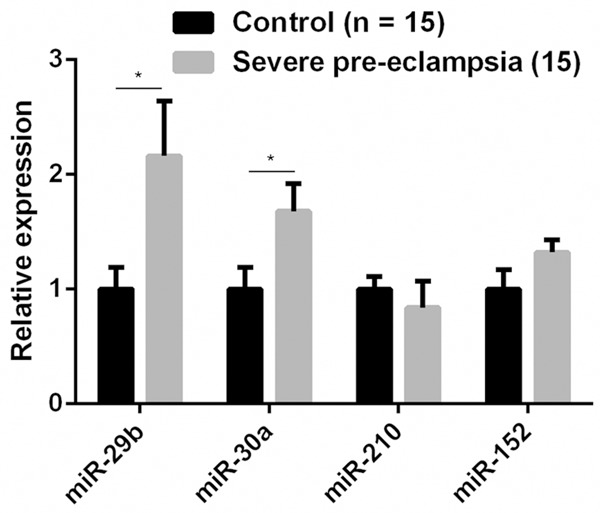
Upregulation of miR-29b in decidua-derived mesenchymal stem cells (dMSCs) from patients with pre-eclampsia. *P < 0.05 when compared to that in normal control subjects.
Overexpression of miR-29b decreased dMSC proliferation
We then examined the role of miR-29b in dMSC viability and proliferation. dMSCs were transfected with various oligonucleotides of miR-29b. Significantly decreased cell viability and proliferation were found at 48 h and 72 h in dMSCs overexpressing miR-29b, whereas significantly increased cell viability and proliferation were observed at 48 h and 72 h in dMSCs transfected with anti-miR-29b (Figure 2).
Figure 2.
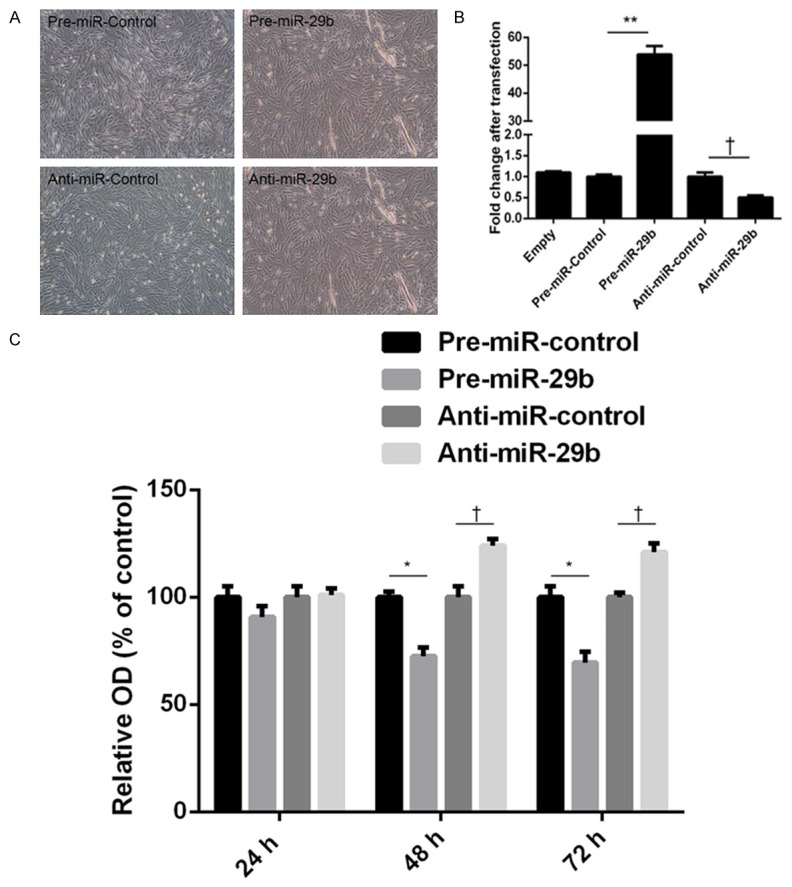
Upregulation of miR-29b decreased the proliferation of decidua-derived mesenchymal stem cells (dMSCs). A. Morphology of dMSCs after transfection with pre-miR-control, pre-miR-29b, anti-miR-control, and anti-miR-29b. B. The expression of miR-29b was quantified by real-time PCR. C. The proliferation of dMSCs was determined by an MTT assay. Significantly decreased dMSC proliferation was found at 48 and 72 h in the miR-29 overexpression group.
MiR-29b overexpressing dMSCs regulate cell migration and angiogenesis
We then examined the effect of miR-29b-overexpressing dMSCs on the migratory capacity and tubule formation ability of HUVECs. dMSCs were transfected with various miR-29b oligonucleotides and CM was collected at 36 h post-transfection. CM was then used as the incubation medium in a transwell system. In HUVECs, significantly decreased migration was observed after incubation with CM from dMSCs transfected with pre-miR-29b, whereas significantly increased migration was found after incubation with CM from dMSCs transfected with anti-miR-29b (Figure 3A). A tubule formation assay was also performed to assess the effect of CM from dMSCs after miR-29b overexpression and inhibition. Significantly decreased and increased tubule formation was observed in HUVECs incubated with CM after miR-29b overexpression and inhibition, respectively.
Figure 3.
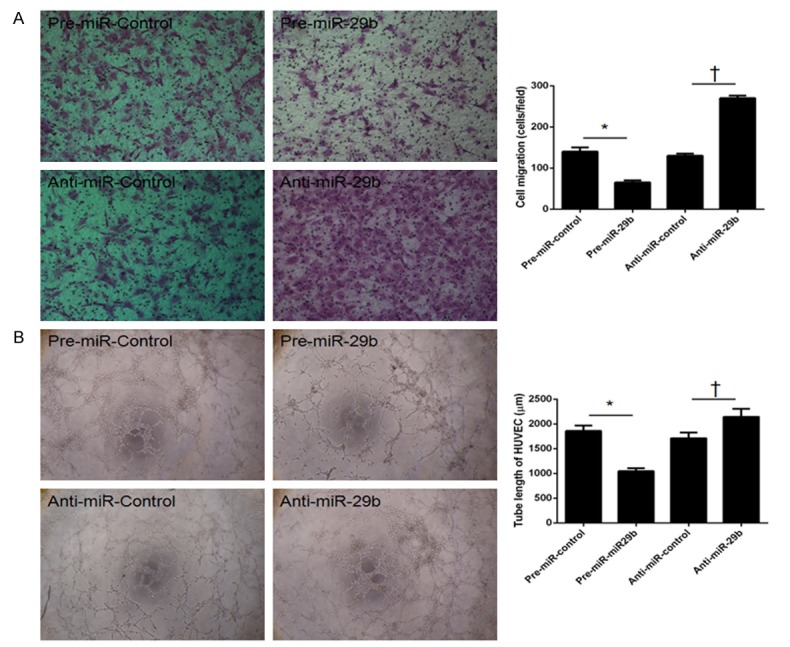
Upregulation of miR-29b in decidua-derived mesenchymal stem cells (dMSCs) decreased cell migration and angiogenesis. dMSCs were transfected with pre-miR-control, pre-miR-29b, anti-miR-control, and anti-miR-29b, and condition medium (CM) from these cells was collected after 36 h. A. HUVEC migration was significantly decreased after incubation with CM from dMSCs transfected with pre-miR-29b, whereas it was significantly increased after incubation with CM from dMSCs transfected with anti-miR-29b. B. Decreased tubule formation in HUVECs was found after incubation with CM from dMSCs transfected with pre-miR-29b, whereas increased tubule formation in HUVECs was observed after incubation with CM from dMSCs transfected with anti-miR-29b. *P < 0.05 when compared to pre-miR-control; †P < 0.05 when compared to anti-miR-control.
MiR-29b targeted HDAC4 at the 3’UTR and decreased its expression in dMSCs
We performed target gene searching of miR-29b using the TargetScan database and the results predicted that HDAC4 has a putative miR-29b binding site within its 3’UTR, which might be a potential target of miR-29b (Figure 4A). To explore the effect of miR-29b on the mRNA and protein levels of HDAC4, dMSCs transfected with pre-miR-29b were examined. The results suggested downregulation of HDAC4 mRNA and protein after transfection (Figure 4C, 4D). A luciferase experiment was also employed to confirm binding of miR-29b to the 3’UTR of HDAC4. Decreased luciferase activity was shown after a partial segment of the 3’UTR of HDAC was cloned into the luciferase reporter vector (Figure 4B).
Figure 4.
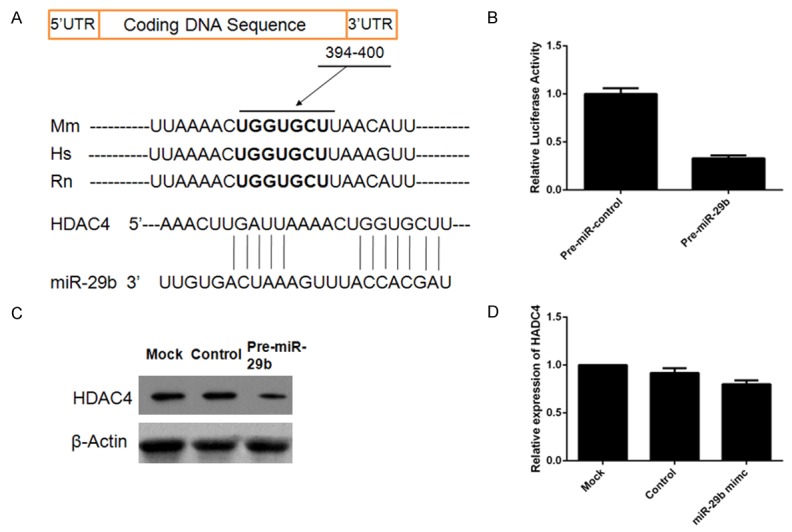
MiR-29b was found to target HDAC4 in decidua-derived mesenchymal stem cells (dMSCs) and reduce HDAC4 expression. A. A putative target site for miR-29b in the 3’-UTR of HDAC4 mRNA predicted by TargetScan. Mm: mouse; Hs: human; Rn: rat. B. Decreased luciferase activity after pre-miR-29b transfection. C. MiR-29 overexpression reduced the protein level of HDAC4. D. MiR-29 overexpression decreased the gene expression of HDAC4. *P < 0.05 when compared to control miRNA.
CM from TSA-treated dMSCs decreased cell migration and angiogenesis
The histone deacetylase inhibitor TSA was employed to verify the role of HDAC4 in dMSCs in the regulation of cell migration and angiogenesis (Figure 5A). Significantly decreased cell migration was observed in HUVECs incubated with CM from TSA-treated dMSCs (Figure 5B). In addition, tubule formation ability was significantly impaired in HUVECs after incubation with CM from TSA-treated dMSCs (Figure 5C).
Figure 5.
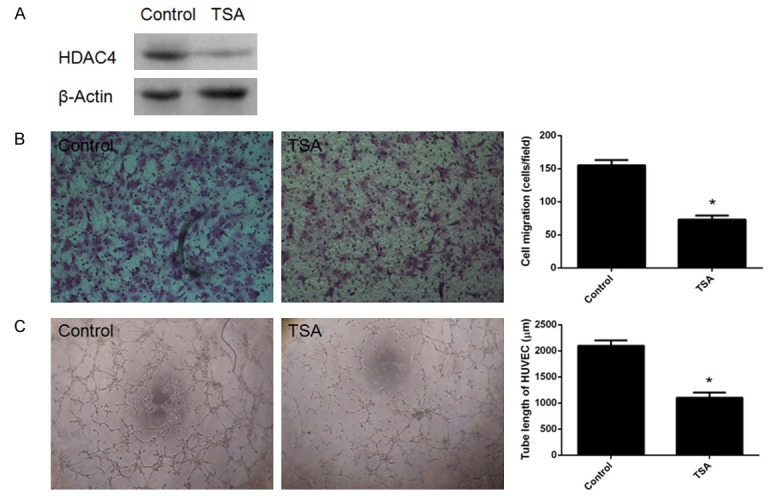
Conditioned medium (CM) from trichostatin A (TSA)-treated decidua-derived mesenchymal stem cells (dMSCs) reduced migration and tubule formation in HUVECs. A. Decreased expression of HDAC4 in dMSCs after treatment with TSA. B. Significantly decreased migration was observed in HUVECs. C. Significantly decreased tubule formation was also identified in HUVECs. *P < 0.05 when compared to control miRNA.
Discussion
In the present study, upregulation of miR-29b was found in dMSCs from patients with PE. Overexpression of miR-29b inhibited the proliferation of dMSCs. Moreover, overexpression of miR-29b in dMSCs reduced the ability of HUVECs to migrate and form blood vessels, and this was thought to be attributed to a diminished paracrine effect, originating from dMSCs, through downregulation of HDAC4. To the best of our knowledge, this is the first study to investigate the role of miR-29b in dMSCs, and these results might provide insight on the pathogenesis of PE.
MSCs represent a heterogeneous population of pluripotent progenitor cells that can be found in almost all tissues [13]. miRNAs have been reported to act as regulatory signals for the function of MSCs [14]. Huang et al [15] showed that miR-204 regulates differentiation of MSCs by decreasing the expression of Runx2. Kim et al [16] suggested that miR-21 regulates adipogenic differentiation by modulating TGF-β signaling in MSCs. As a systemic disease, the dysfunction of maternal endothelial cells is involved in the pathogenesis of PE [17]. The decidua is a type of highly modified endometrium, and proper formation and function of this tissue are required for normal pregnancy [18]. Previous studies have shown that MSCs can be isolated from decidua tissues and that they are involved in the pathogenesis of PE [5,11]. Moreover, differential expression of miRNAs was found between the placenta from PE patients and those of normal pregnancies [19]. In this study, we confirmed the differential expression of miRNAs in dMSCs of PE patients and normal control subjects. Among these miRNAs, miR-29b exhibited the largest fold change. We then overexpressed miR-29b in dMSCs and found that proliferation was significantly decreased after the transfection of miR-29b.
During the past few years, the paracrine effect of MSCs has been the emphasis of many recent studies [20,21]. Acceding with previous studies [22,23], many growth factors and proteases can be secreted by MSCs, including vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), insulin-like growth factor (IGF)-1, stromal cell-derived factor (SDF), basic fibroblast growth factor (bFGF), matrix metalloproteinases (MMP), transforming growth factor (TGF)-β, and platelet derived growth factor (PDGF). Moreover, differential expression of cytokines has been observed between dMSCs from patients with PE and those with normal pregnancies [24]. Therefore, we examined the effect of miR-29b overexpression in dMSCs on the microenvironment of the placenta. CM from these dMSCs and their effect on the migration and angiogenesis of HUVECs were tested. The results demonstrated decreased migration and tubule formation in HUVECs after incubation with CM collected from miR-29b-overexpressing dMSCs.
HDAC4 is a member of the class IIa histone deacetylase family and previous studies have shown that it is involved in bone development [25] and promotes TGF-β1 induced chondrogenesis in synovium-derived stem cells and inhibits chondrogenically differentiated stem cell hypertrophy [26]. Most recently, Zhu et al demonstrated alteration of HDAC (such as HDAC4, HDAC5, and HDAC6) activity in placental derived MSCs [27]. However, the exact role of HDACs in PE had not previously been explored. By using the online database TargetScan, we identified HDACs to be a potential target gene of miR-29b and showed that HDAC4 regulated the paracrine behavior of dMSCs; this was verified by the observation that HUVECs incubated with CM from TSA-treated dMSCs exhibited decreased migration and tubule formation.
In conclusion, our results demonstrated that upregulation of miR-29b in dMSCs plays an important role in the paracrine effect of dMSCs and that this might be involved in the development of PE. Our study proposed the existence of a new molecular event that might explain the pathogenesis of disease.
Disclosure of conflict of interest
None.
References
- 1.Uzan J, Carbonnel M, Piconne O, Asmar R, Ayoubi JM. Pre-eclampsia: pathophysiology, diagnosis, and management. Vasc Health Risk Manag. 2011;7:467–474. doi: 10.2147/VHRM.S20181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sibai B, Dekker G, Kupferminc M. Preeclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 3.Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, Noel D, Jorgensen C. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–3844. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 4.Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini FR. Concise review: dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011;29:11–19. doi: 10.1002/stem.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu L, Zhao G, Fan H, Zhao X, Li P, Wang Z, Hu Y, Hou Y. Mesenchymal stem cells ameliorate th1-induced pre-eclampsia-like symptoms in mice via the suppression of tnf-alpha expression. PLoS One. 2014;9:e88036. doi: 10.1371/journal.pone.0088036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartel DP. Micrornas: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, Chavakis E, Potente M, Tjwa M, Urbich C, Zeiher AM, Dimmeler S. Microrna-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 8.Parmacek MS. Microrna-modulated targeting of vascular smooth muscle cells. J Clin Invest. 2009;119:2526–2528. doi: 10.1172/JCI40503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu C, Zhou Y, Zhang Z. Mir-210: an important player in the pathogenesis of preeclampsia? J Cell Mol Med. 2012;16:943–944. doi: 10.1111/j.1582-4934.2011.01370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Diao Z, Su L, Sun H, Li R, Cui H, Hu Y. Microrna-155 contributes to preeclampsia by down-regulating cyr61. Am J Obstet Gynecol. 2010;202:466.e1–7. doi: 10.1016/j.ajog.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Fan H, Zhao G, Liu D, Du L, Wang Z, Hu Y, Hou Y. Mir-16 inhibits the proliferation and angiogenesis-regulating potential of mesenchymal stem cells in severe pre-eclampsia. FEBS J. 2012;279:4510–4524. doi: 10.1111/febs.12037. [DOI] [PubMed] [Google Scholar]
- 12.Ji Y, Strawn TL, Grunz EA, Stevenson MJ, Lohman AW, Lawrence DA, Fay WP. Multifaceted role of plasminogen activator inhibitor-1 in regulating early remodeling of vein bypass grafts. Arterioscler Thromb Vasc Biol. 2011;31:1781–1787. doi: 10.1161/ATVBAHA.111.228767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collino F, Bruno S, Deregibus MC, Tetta C, Camussi G. Micrornas and mesenchymal stem cells. Vitam Horm. 2011;87:291–320. doi: 10.1016/B978-0-12-386015-6.00033-0. [DOI] [PubMed] [Google Scholar]
- 15.Huang J, Zhao L, Xing L, Chen D. Microrna-204 regulates runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells. 2010;28:357–364. doi: 10.1002/stem.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim YJ, Hwang SJ, Bae YC, Jung JS. Mir-21 regulates adipogenic differentiation through the modulation of tgf-beta signaling in mesenchymal stem cells derived from human adipose tissue. Stem Cells. 2009;27:3093–3102. doi: 10.1002/stem.235. [DOI] [PubMed] [Google Scholar]
- 17.Cudihy D, Lee RV. The pathophysiology of pre-eclampsia: current clinical concepts. J Obstet Gynaecol. 2009;29:576–582. doi: 10.1080/01443610903061751. [DOI] [PubMed] [Google Scholar]
- 18.Winn VD, Gormley M, Fisher SJ. The impact of preeclampsia on gene expression at the maternal-fetal interface. Pregnancy Hypertens. 2011;1:100–108. doi: 10.1016/j.preghy.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Y, Li P, Hao S, Liu L, Zhao J, Hou Y. Differential expression of micrornas in the placentae of Chinese patients with severe preeclampsia. Clin Chem Lab Med. 2009;47:923–929. doi: 10.1515/CCLM.2009.228. [DOI] [PubMed] [Google Scholar]
- 20.Xiang MX, He AN, Wang JA, Gui C. Protective paracrine effect of mesenchymal stem cells on cardiomyocytes. J Zhejiang Univ Sci B. 2009;10:619–624. doi: 10.1631/jzus.B0920153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kemp KC, Hows J, Donaldson C. Bone marrow-derived mesenchymal stem cells. Leuk Lymphoma. 2005;46:1531–1544. doi: 10.1080/10428190500215076. [DOI] [PubMed] [Google Scholar]
- 23.Wang PP, Wang JH, Yan ZP, Hu MY, Lau GK, Fan ST, Luk JM. Expression of hepatocyte-like phenotypes in bone marrow stromal cells after hgf induction. Biochem Biophys Res Commun. 2004;320:712–716. doi: 10.1016/j.bbrc.2004.05.213. [DOI] [PubMed] [Google Scholar]
- 24.Hwang JH, Lee MJ, Seok OS, Paek YC, Cho GJ, Seol HJ, Lee JK, Oh MJ. Cytokine expression in placenta-derived mesenchymal stem cells in patients with pre-eclampsia and normal pregnancies. Cytokine. 2010;49:95–101. doi: 10.1016/j.cyto.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Hug BA. Hdac4: a corepressor controlling bone development. Cell. 2004;119:448–449. doi: 10.1016/j.cell.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 26.Pei M, Chen D, Li J, Wei L. Histone deacetylase 4 promotes tgf-beta1-induced synoviumderived stem cell chondrogenesis but inhibits chondrogenically differentiated stem cell hypertrophy. Differentiation. 2009;78:260–268. doi: 10.1016/j.diff.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Zhu Y, Song X, Han F, Li Y, Wei J, Liu X. Alteration of histone acetylation pattern during long-term serum-free culture conditions of human fetal placental mesenchymal stem cells. PLoS One. 2015;10:e117068. doi: 10.1371/journal.pone.0117068. [DOI] [PMC free article] [PubMed] [Google Scholar]


