Abstract
In the tumor microenvironment coexisting with Hashimoto’s thyroiditis (HT), cytokines secreted by the tumor cells, stroma cells, or immune cells play a critical role in the regulation of tumor growth, invasion, and metastasis. The present study aims to understand cytokine production from cancerous tissues (CT), para-cancerous tissues (PT), and serum in patients with papillary thyroid carcinoma (PTC) with or without accompanying HT. Using a multiplexed human cytokine assay, we found that nine cytokines, including Interleukin-1alpha (IL-1α), Interleukin-1beta (IL-1β), Interleukin-12p70 (IL-12p70), Interleukin-8 (IL-8), Interferon-inducible protein-10 (IP-10), Monocyte chemoattractant protein-1 (MCP-1), Macrophage inflammatory protein-1alpha (MIP-1α), Macrophage inflammatory protein-1beta (MIP-1β), and soluble E-selectin (sE-Selectin), showed significantly higher expression in para-cancerous tissues of HT+PTC compared with HT-PTC (P<0.05). In addition, H&E staining showed immune cell infiltration in PT but not in CT. Moreover para-cancerous tissues of HT+PTC patients produced more Interferon-alpha (IFN-α) (P=0.048) and Interferon-gamma (IFN-γ) (P=0.004) compared with cancerous tissues, and production of Intercellular cell adhesion molecule-1 (ICAM-1) was significantly higher in CT than PT both in HT+PTC (P=0.001) and HT-PTC (P=0.012) patients. To conclude, autoimmune HT was found to affect the cytokine profiles in patients with PTC by stimulating secretion of Th1-type cytokines and chemokines, but further studies are needed to determine the significance of these findings and to reveal the exact mechanism of the interactions between chemokines and cytokines in the pathogenesis of HT and PTC.
Keywords: Hashimoto’s thyroiditis, papillary thyroid carcinoma, cytokine, chemokine
Introduction
Hashimoto’s thyroiditis (HT) is the most frequent autoimmune disease and is characterized by a cellular immune response with lymphatic infiltration of the thyroid gland by T and B cells, as well as a humoral immune response leading to specific antibody production of thyroglobulin (TgAb) and thyroperoxidase (TPOAb) and destruction of thyroid follicles.
Thyroid cancer is the most common endocrine malignancy and papillary thyroid carcinoma (PTC) accounts for 85% to 90% of cases. Moreover, the incidence of PTC has increased 3-fold over the last 3 decades [1] and a parallel increase in the incidence of autoimmune thyroid diseases such as HT has been observed. Because coexistence of HT and PTC in the thyroid gland is often found in the clinic, debate about the relationship between these diseases has continued since Dailey first proposed it in 1955 [2].
The connection between a chronic inflammatory immune response and tumorigenesis has been extensively investigated during the past decade and some mechanisms have been elucidated, such as the association between viral hepatitis B or C and liver cancer and between Crohn’s disease and colon cancer. Likewise, a retrospective Chinese cohort study showed that PTC with HT was associated with increased prevalence of multi-focal disease and capsular invasion [3], suggesting the need to further analyze the thyroid microenvironment of HT.
In the tumor microenvironment coexisting with HT, cytokines secreted by the tumor cells, stroma cells, or immune cells play a critical role in the regulation of tumor growth, invasion, and metastasis. The present study aims to understand cytokine production from cancerous tissues (CT), para-cancerous tissues (PT), and serum in patients with PTC with or without accompanying HT.
Materials and methods
Patient selection
This study was approved by the local ethical committees and all patients provided informed consent before blood collection and surgery. Patients who were diagnosed with PTC, irrespective of accompanying HT, and underwent thyroidectomy or lobectomy between May 2016 and October 2016 at the Department of Head and Neck Surgery, Sir Run Run Shaw Hospital, were identified as eligible for the study. After the operation, histological diagnosis of PTC and HT was confirmed by two experienced pathologists from the Department of Pathology of the same hospital.
The patients involved in this study met the following criteria: no previous thyroid surgery, pregnancy, infection, other autoimmune disease, or other malignancies at the time of the investigation. All patients were selected randomly. Finally, 22 patients were enrolled in the study: 11 women and 10 men aged from 24 to 67 years with average age of 43. Patients with PTC were divided into two groups: PTC patients with Hashimoto’s thyroiditis (HT+PTC group, 13 patients) and PTC patients without coexisting Hashimoto’s thyroiditis (HT-PTC group, 9 patients). Because the tumor sizes in some enrolled patients were too small for analysis, we examined 18 cancerous tissues and serum samples (12 HT+PTC, 6 HT-PTC).
Sample preparation
Serum and tissue samples were collected from each patient. Specifically, for serum sample preparation, fasting blood samples were collected 1 day before operation and the blood was allowed to clot for 20-30 min at 20-25°C. The clotted blood samples were centrifuged at 1000×g for 10 min at 20-25°C and the serum fraction was collected and stored at -80°C. For tissue sample preparation, papillary thyroid carcinoma tissues and para-cancerous tissues (2 cm from cancerous tissues), which were confirmed to contain no cancer cells by two experienced pathologists, were collected during surgery and immediately placed in liquid nitrogen until analysis.
Hematoxylin-eosin staining
Thyroid carcinoma tissues and nearby normal tissues were sent immediately to the Department of Pathology. Tissues were fixed in 10% neutral buffered formalin at 4°C overnight, dehydrated, and embedded in paraffin. Section slides were stained with hematoxylin-eosin (H&E) for pathological diagnosis [4].
Cytokine quantification
Cytokine levels in tissue homogenates and serum were measured using the Procarta Plex Multiplex Immunoassay kit (Affymetrix, eBiosciences, USA) according to the user manual. This multiplexed protein measurement used magnetic bead technology from Luminex to measure concentrations of ICAM-1, IFN-γ, IFN-α, IL-1α, IL-1β, IL-12p70, IL-8, IP-10, MCP-1, MIP-1α, MIP-1β, and sE-Selectin.
Statistical analysis
Data were analyzed by SPSS software version 23.0 (SPSS, Inc., Chicago, IL, USA). Statistical analyses were performed using the t test. Quantitative data are displayed as the mean and standard deviation (SD). Differences with p values <0.05 were defined as statistically significant.
Results
Abundant immune cells infiltrated into para-cancerous tissues of HT+PTC patients
H&E staining results showed that immune cells only infiltrated into the para-cancerous tissues and not the cancerous tissues in HT+PTC patients. Furthermore, some lymphoid germinal centers, which can often be seen as secondary lymphoid follicles (LF), were observed in the adjacent non-neoplastic parenchyma of PTC patients with severe HT (Figure 1A and 1B). No immune cells could be found in para-cancerous tissues and cancerous tissues of HT-PTC patients by optical microscopy (Figure 1C and 1D).
Figure 1.
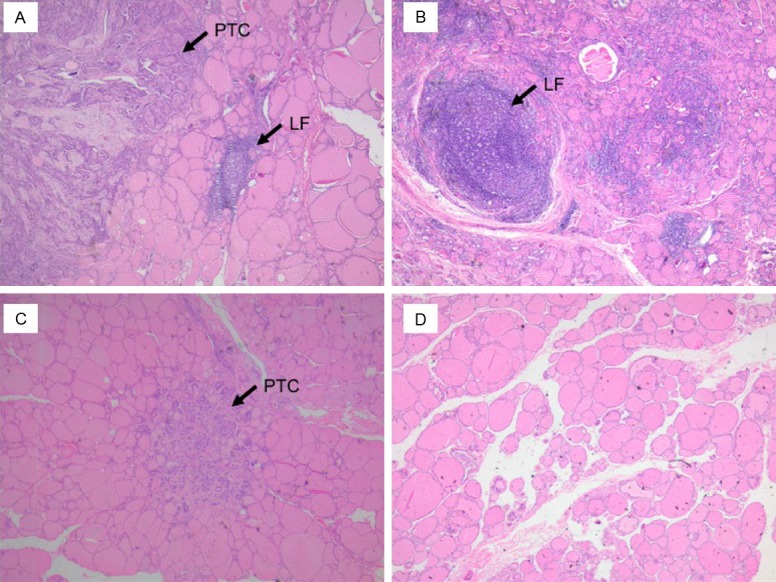
Representative hematoxylin and eosin-stained sections of cancerous tissues and para-cancerous tissues (40× magnification). A. Cancerous tissue (HT+PTC): few immune cells; B. Para-cancerous tissue (HT+PTC): immune cells infiltrated into para-cancerous tissues with distinct lymphoid follicular (LF) growth; C. Cancerous tissue (HT-PTC): very few infiltrated immune cells; D. Para-cancerous tissue (HT-PTC): almost no infiltrated immune cells.
Increased levels of IL-1α, IL-1β, IL-12p70, IL-8, IP-10, MCP-1, MIP-1α, MIP-1β, and sE-Selectin in para-cancerous tissues of HT+PTC patients
Levels of 12 cytokines in cancerous tissues, para-cancerous tissues, and serum from PTC patients with or without HT were analyzed. The level of 9 cytokines (IL-1α, IL-1β, IL-12p70, IL-8, IP-10, MCP-1, MIP-1α, MIP-1β, and sE-Selectin) in para-cancerous tissues was significantly higher (P<0.05) in HT+PTC patients than in HT-PTC patients (Table 1; Figure 2); however, there was no difference (P≥0.05) between the two groups for cytokine levels in cancerous tissues and serum, which might be explained by the small sample size and high inter-individual variation among patients.
Table 1.
Cytokine concentrations in para-cancerous tissues of PTC patients with or without Hashimoto thyroiditis and differences between the two groups
| Cytokine | HT+PTC (n=13) x ± SD | HT-PTC (n=9) x ± SD | Value of P |
|---|---|---|---|
| ICAM-1 | 76012.5 ± 22340.5 | 75850.7 ± 32882.2 | 0.989 |
| IFN-γ | 3.7 ± 1.8 | 2.2 ± 1.4 | 0.064 |
| IFN-α | 1.8 ± 0.5 | 1.6 ± 0.6 | 0.382 |
| IL-1α | 17.4 ± 5.2 | 9.8 ± 3.6 | 0.001 |
| IL-1β | 25.2 ± 7.4 | 2.1 ± 1.7 | 0.001 |
| IL-12p70 | 6.8 ± 3.2 | 4.2 ± 1.9 | 0.026 |
| IL-8 | 190.1 ± 68.0 | 104.7 ± 41.2 | 0.003 |
| IP-10 | 518.0 ± 168.9 | 265.6 ± 65.5 | 0.001 |
| MCP-1 | 3097.7 ± 1244.2 | 2109.4 ± 653.0 | 0.026 |
| MIP-1α | 83.7 ± 19.8 | 47.5 ± 18.9 | 0.001 |
| MIP-1β | 432.2 ± 164.8 | 223.8 ± 118.7 | 0.004 |
| sE-Selectin | 7792.5 ± 2219.0 | 4100.1 ± 1677.3 | 0.001 |
Figure 2.
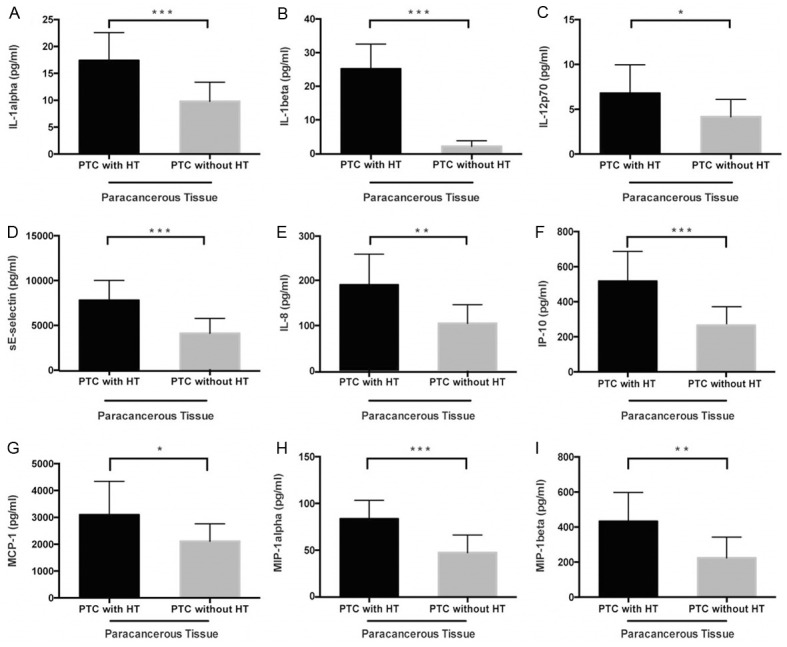
Higher expression levels of (A) IL-1α, (B) IL-1β, (C) IL-12p70, (D) IL-8, (E) IP-10, (F) MCP-1, (G) MIP-1α, (H) MIP-1β and (I) sE-Selectin in para-cancerous tissues of PTC patients with HT compared with those without HT (t-test, *P<0.05, **P≤0.01, ***P≤0.001).
Differences in cytokine levels between cancerous tissues and para-cancerous tissues of HT+PTC and HT-PTC groups
Para-cancerous tissues of HT+PTC patients produced more IFN-α (P=0.048) and IFN-γ (P=0.004) compared with cancerous tissues. In addition, production of ICAM-1 was significantly higher in cancerous tissues than para-cancerous tissues both in HT+PTC (P=0.001) and HT-PTC (P=0.012) patients (Figure 3; Table 2).
Figure 3.
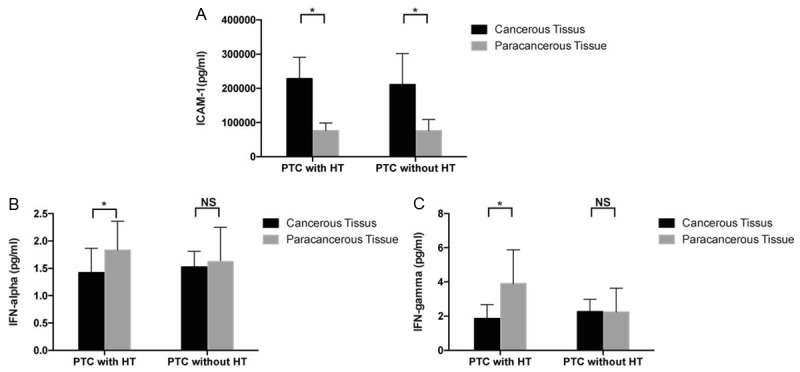
Expression levels of ICAM-1 (A), IFN-α (B), and IFN-γ (C) in HT+PTC and HT-PTC groups (t-test, *P<0.05, **P≤0.01, ***P≤0.001).
Table 2.
Cytokine concentrations in cancerous tissues and para-cancerous tissues of PTC patients with or without Hashimoto thyroiditis and differences between cancerous tissues and para-cancerous tissues
| Cytokine | PTC with HT | PTC without HT | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Cancerous Tissue (n=12) x ± SD | Para-cancerous Tissue (n=13) x ± SD | Value of P | Cancerous Tissue (n=6) x ± SD | Para-cancerous Tissue (n=9) x ± SD | Value of P | |
| ICAM-1 | 228184.8 ± 62529.1 | 76012.5 ± 22340.5 | 0.001 | 211812.7 ± 89759.9 | 75850.7 ± 32882.2 | 0.012 |
| IFN-γ | 1.9 ± 0.8 | 3.9 ± 2.0 | 0.004 | 2.3 ± 0.7 | 2.2 ± 1.4 | 0.974 |
| IFN-α | 1.4 ± 0.4 | 1.8 ± 0.5 | 0.048 | 1.5 ± 0.3 | 1.6 ± 0.6 | 0.770 |
| IL-1α | 21.7 ± 11.7 | 18.2 ± 9.4 | 0.415 | 19.0 ± 13.6 | 10.9 ± 6.1 | 0.136 |
| IL-1β | 18.6 ± 19.1 | 25.2 ± 7.4 | 0.261 | 10.9 ± 10.5 | 2.1 ± 1.7 | 0.094 |
| IL-12p70 | 5.6 ± 1.4 | 6.8 ± 3.2 | 0.218 | 5.8 ± 2.1 | 4.2 ± 1.9 | 0.144 |
| IL-8 | 289.2 ± 173.2 | 190.1 ± 68.0 | 0.085 | 191.1 ± 105.1 | 104.7 ± 41.2 | 0.103 |
| IP-10 | 414.4 ± 272.7 | 518.0 ± 168.9 | 0.261 | 317.9 ± 193.1 | 265.6 ± 65.5 | 0.548 |
| MCP-1 | 2257.6 ± 1024.2 | 3097.7 ± 1244.2 | 0.080 | 2308.5 ± 810.0 | 2109.4 ± 653.0 | 0.607 |
| MIP-1α | 97.5 ± 26.8 | 83.7 ± 19.8 | 0.153 | 148.8 ± 171.6 | 47.5 ± 18.9 | 0.208 |
| MIP-1β | 628.9 ± 702.3 | 432.2 ± 164.8 | 0.362 | 384.6 ± 235.3 | 223.8 ± 118.7 | 0.168 |
| sE-Selectin | 6783.0 ± 2984.7 | 7792.5 ± 2219.0 | 0.345 | 6763.0 ± 2831.6 | 4100.1 ± 1677.3 | 0.038 |
Discussion
Cytokines are multifunctional cell signaling molecules that are produced in response to inflammation, infection, and cellular trauma [5,6]. They may affect the cells they were secreted from, other cells nearby, or even influence the whole body. These actions play a significant role in maintaining homeostasis via complex interactions of the nervous, endocrine, and immune systems [7,8]. In addition, cytokines are thought to mediate the origination and perpetuation of autoimmune thyroid disease, especially Hashimoto’s thyroiditis. Moreover, some cytokines have a close correlation with the initiation and progression of cancer [9,10].
It is traditionally assumed that the immune functional imbalance between T-helper 1 cell (Th1) and T-helper 2 cell (Th2) leads to tumor growth. In the report of Phenekos, the preferential expression of IL-2, IFN-γ, IL-12, and IL-18, a Th1 pattern of immune response, which is characteristic of cellular immunity, is dominant in HT. In the same way, our study showed that the HT+PTC patients presented higher levels of IL-1α, IL-1β, and IL-12p70, which are also Th1 pattern cytokines, in para-cancerous tissues compared with HT-PTC patients (P=0.001, P=0.001, P=0.026, respectively). The preferential expression of IL-12 can promote CD4+T lymphocytes to differentiate into Th1 cells. IL-1 has been found to stimulate thyroid cell proliferation and modify thyroid epithelial tightness by altering the expression, localization, and organization of junction proteins, confirming the important role played by IL-1β in the process of carcinogenesis [11,12]. However, IL-1β plays a role in suppressing proliferation and reducing the invasive potential of human PTC cells [13]. We think this conflict actually accord with the clinical findings: PTC coexisting with HT is strongly associated with tumor multifocality, the absence of extrathyroidal extension, absence of lymph node metastasis, and high recurrence-free survival rates [14].
It has been suggested that E-selectin is related to the mechanism of neoplastic progression and metastasis [15]. Some investigators have reported a strong association between E-selectin expression and the presence of extrathyroidal extension [16], which correlated with the features of HT. Chronic inflammation probably contributes to tumor generation through increasing the secretion of E-selectin.
Chemokines are a family of small cytokines or signaling proteins that act as chemo-attractants to guide cell migration. Chemokines are functionally divided into two groups: one is the homeostatic chemokine, which is produced in certain tissues and is responsible for basal leukocyte migration; the other is the inflammatory chemokine (IL-8, IP-10, MCP-1, MIP-1α, MIP-1β), which is produced under pathological conditions (or pro-inflammatory stimuli such as IL-1 or viruses) and actively participates in the inflammatory response by attracting immune cells to the site of inflammation.
According to the arrangement of Cysteine on N-terminal amino acid, IL-8 and IP-10 are members of the CXC chemokine supergene family that attract neutrophils to the sites of inflammation [17], and MCP-1, MIP-1α, MIP-1β belong to the CC chemokine supergene family that attract monocytes and macrophages to the sites of inflammation [18]. Thus, MCP-1, MIP-1α, and MIP-1β were considered to induce monocytes to leave the bloodstream and enter thyroid tissues of PTC patients with HT to become tissue macrophages [19]. In addition, IL-8 and IP-10 induce neutrophils in HT+PTC patients, followed by inflammatory response and tissue damage [20].
In our research, mean production levels of chemokines (IL-8, IP-10, MCP-1, MIP-1α, MIP-1β) tended to be higher in thyroid para-cancerous tissues of HT+PTC patients compared with HT-PTC (P=0.003, P=0.001, P=0.026, P=0.001, P=0.001, respectively). Some researchers indicated that IP-10 might be a marker of host immune response due to a significant increase of IP-10 in Hashimoto’s thyroiditis tissue specimens [21,22], Visciano et al. proposed that IL-8 promoted thyroid carcinoma generation and angiogenesis [23]. Besides, the previous study revealed the effect of MIP-1 and MCP-1 on the development of autoimmune diseases, which was in agreement with our present work. These cytokines have been found in synoviocytes in rheumatoid arthritis and were thought to play a role in recruiting monocytes to joint inflammation (Villiger, 1992) [24]. Kemp confirmed expression of MCP-1, MIP-1α, MIP-1β, and IP-10 in all Hashimoto’s disease and most Graves’ disease thyroid specimens, whereas very little expression was detected in non-autoimmune goiter samples [25]. Although the present research indicated a high expression of these chemokines in patients with HT, their association with papillary thyroid carcinoma remains unknown.
The expression of ICAM-1 produced by thyroid cells can be enhanced by IL-1 [11], consistent with our experimental results. Buitrago et al. [26] showed that ICAM-1 was upregulated in PTC at the levels of both gene and protein expression. Moreover, this upregulated expression correlated with aggressive tumor features such as BRAF V600E mutation, extrathyroidal extension, and lymph node metastasis. The biological activity of IFN-γ is conventionally linked to cytostatic and cytotoxic and antitumor mechanisms during the cell-mediated immune response [27]. Regarding tumor immunity, it is generally accepted that IFN-γ has an important role in tumor immune surveillance. In our study higher expression of INF-γ in para-cancerous tissue in HT+PTC indicated that the autoimmune condition might be beneficial for the host to surveille and constrict the tumor growth.
In conclusion, autoimmune HT was found to affect the cytokine profiles in patients with PTC by stimulating the secretion of Th1-type cytokines and chemokines. As most cytokines appear to have contradictory functions in tumor immune surveillance and autoimmunity, the exact regulatory mechanisms of cytokines and/or chemokines between HT and PTC remain unclear. Further studies are needed to determine the significance of these findings and elucidate the interaction between chemokines and cytokines in the pathogenesis of PTC coexisting with HT.
Acknowledgements
This work was supported by College Students’ Scientific and Technological Innovation Projects of Zhejiang Province (No. 2016R401259), and the Key project from the Health and Family Planning Commission of Zhejiang Province (No. 201510112).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Dailey ME, Lindsay S, Skahen R. Relation of thyroid neoplasms to Hashimoto disease of the thyroid gland. AMA Arch Surg. 1955;70:291–297. doi: 10.1001/archsurg.1955.01270080137023. [DOI] [PubMed] [Google Scholar]
- 3.Zhu F, Shen YB, Li FQ, Fang Y, Hu L, Wu YJ. The effects of hashimoto thyroiditis on lymph node metastases in unifocal and multifocal papillary thyroid carcinoma: a retrospective Chinese cohort study. Medicine (Baltimore) 2016;95:e2674. doi: 10.1097/MD.0000000000002674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehdizadeh R, Parizadeh MR, Khooei AR, Mehri S, Hosseinzadeh H. Cardioprotective effect of saffron extract and safranal in isoproterenol-induced myocardial infarction in wistar rats. Iran J Basic Med Sci. 2013;16:56–63. [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy RL, Jones TH. Cytokines in endocrinology: their roles in health and in disease. J Endocrinol. 1991;129:167–178. doi: 10.1677/joe.0.1290167. [DOI] [PubMed] [Google Scholar]
- 6.Jones TH. Interleukin-6 an endocrine cytokine. Clin Endocrinol (Oxf) 1994;40:703–713. doi: 10.1111/j.1365-2265.1994.tb02502.x. [DOI] [PubMed] [Google Scholar]
- 7.Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 8.Bethin KE, Vogt SK, Muglia LJ. Interleukin-6 is an essential, corticotropin-releasing hormone-independent stimulator of the adrenal axis during immune system activation. Proc Natl Acad Sci U S A. 2000;97:9317–9322. doi: 10.1073/pnas.97.16.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diamant M, Kayser L, Rasmussen AK, Bech K, Feldt-Rassmussen U. Interleukin-6 production by thyroid epithelial cells. Enhancement by interleukin-1. Autoimmunity. 1991;11:21–26. doi: 10.3109/08916939108994704. [DOI] [PubMed] [Google Scholar]
- 10.Zheng RQ, Abney E, Chu CQ, Field M, Grubeck-Loebenstein B, Maini RN, Feldmann M. Detection of interleukin-6 and interleukin-1 production in human thyroid epithelial cells by non-radioactive in situ hybridization and immunohistochemical methods. Clin Exp Immunol. 1991;83:314–319. doi: 10.1111/j.1365-2249.1991.tb05634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mine M, Tramontano D, Chin WW, Ingbar SH. Interleukin-1 stimulates thyroid cell growth and increases the concentration of the c-myc proto-oncogene mRNA in thyroid follicular cells in culture. Endocrinology. 1987;120:1212–1214. doi: 10.1210/endo-120-3-1212. [DOI] [PubMed] [Google Scholar]
- 12.Kammoun-Krichen M, Bougacha-Elleuch N, Mnif M, Bougacha F, Charffedine I, Rebuffat S, Rebai A, Glasson E, Abid M, Ayadi F, Peraldi-Roux S, Ayadi H. IL-1beta a potential factor for discriminating between thyroid carcinoma and atrophic thyroiditis. Eur Cytokine Netw. 2012;23:101–106. doi: 10.1684/ecn.2012.0312. [DOI] [PubMed] [Google Scholar]
- 13.Ellyard JI, Simson L, Parish CR. Th2-mediated anti-tumour immunity: friend or foe? Tissue Antigens. 2007;70:1–11. doi: 10.1111/j.1399-0039.2007.00869.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Kim Y, Choi JW, Kim YS. The association between papillary thyroid carcinoma and histologically proven Hashimoto’s thyroiditis: a meta-analysis. Eur J Endocrinol. 2013;168:343–349. doi: 10.1530/EJE-12-0903. [DOI] [PubMed] [Google Scholar]
- 15.Hiratsuka S, Goel S, Kamoun WS, Maru Y, Fukumura D, Duda DG, Jain RK. Endothelial focal adhesion kinase mediates cancer cell homing to discrete regions of the lungs via E-selectin up-regulation. Proc Natl Acad Sci U S A. 2011;108:3725–3730. doi: 10.1073/pnas.1100446108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miccoli P, Torregrossa L, Borrelli N, Materazzi G, Cacciato Insilla A, Miccoli M, Basolo F. E-selectin expression and BRAF status in papillary thyroid carcinomas: correlation with clinicopathologic features. Surgery. 2014;156:1550–1557. doi: 10.1016/j.surg.2014.08.049. discussion 1557-1558. [DOI] [PubMed] [Google Scholar]
- 17.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines--CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 18.Leonard EJ, Yoshimura T. Human monocyte chemoattractant protein-1 (MCP-1) Immunol Today. 1990;11:97–101. doi: 10.1016/0167-5699(90)90035-8. [DOI] [PubMed] [Google Scholar]
- 19.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ajjan RA, Watson PF, McIntosh RS, Weetman AP. Intrathyroidal cytokine gene expression in Hashimoto’s thyroiditis. Clin Exp Immunol. 1996;105:523–528. doi: 10.1046/j.1365-2249.1996.d01-784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Lopez MA, Sancho D, Sanchez-Madrid F, Marazuela M. Thyrocytes from autoimmune thyroid disorders produce the chemokines IP-10 and Mig and attract CXCR3+ lymphocytes. J Clin Endocrinol Metab. 2001;86:5008–5016. doi: 10.1210/jcem.86.10.7953. [DOI] [PubMed] [Google Scholar]
- 22.Antonelli A, Fallahi P, Rotondi M, Ferrari SM, Romagnani P, Grosso M, Ferrannini E, Serio M. Increased serum CXCL10 in Graves’ disease or autoimmune thyroiditis is not associated with hyper- or hypothyroidism per se, but is specifically sustained by the autoimmune, inflammatory process. Eur J Endocrinol. 2006;154:651–658. doi: 10.1530/eje.1.02137. [DOI] [PubMed] [Google Scholar]
- 23.Visciano C, Liotti F, Prevete N, Cali G, Franco R, Collina F, de Paulis A, Marone G, Santoro M, Melillo RM. Mast cells induce epithelial-tomesenchymal transition and stem cell features in human thyroid cancer cells through an IL-8-Akt-Slug pathway. Oncogene. 2015;34:5175–5186. doi: 10.1038/onc.2014.441. [DOI] [PubMed] [Google Scholar]
- 24.Villiger PM, Terkeltaub R, Lotz M. Production of monocyte chemoattractant protein-1 by inflamed synovial tissue and cultured synoviocytes. J Immunol. 1992;149:722–727. [PubMed] [Google Scholar]
- 25.Kemp EH, Metcalfe RA, Smith KA, Woodroofe MN, Watson PF, Weetman AP. Detection and localization of chemokine gene expression in autoimmune thyroid disease. Clin Endocrinol (Oxf) 2003;59:207–213. doi: 10.1046/j.1365-2265.2003.01824.x. [DOI] [PubMed] [Google Scholar]
- 26.Buitrago D, Keutgen XM, Crowley M, Filicori F, Aldailami H, Hoda R, Liu YF, Hoda RS, Scognamiglio T, Jin M, Fahey TJ 3rd, Zarnegar R. Intercellular adhesion molecule-1 (ICAM-1) is upregulated in aggressive papillary thyroid carcinoma. Ann Surg Oncol. 2012;19:973–980. doi: 10.1245/s10434-011-2029-0. [DOI] [PubMed] [Google Scholar]
- 27.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]


