Abstract
Colorectal cancer (CRC) is the second most common cancer in the world. The incidence of this cancer is increasing in the developing countries. Mechanism of CRC tumorigenesis has been widely studied at the molecular levels, and has been recently proposed microRNAs as novel players in CRC. It has been reported that microRNA-138-5p (miR-138-5p) play key roles in different kinds of human cancers. However, the roles and underlying molecular mechanisms of miR-138-5p in CRC have not been adequately elucidated. Thus, the aim of the present study was to investigate the roles and possible regulatory mechanisms of miR-138-5p in CRC. In this study, we demonstrated that miR-138-5p was significantly down-regulated in CRC tissue samples and cell lines. Functional analyses indicated that the overexpression of miR-138-5p significantly delayed cell proliferation, reduced colony formation and increased apoptosis in CRC cell lines. Moreover, human telomerase reverse transcriptase (hTERT), an important oncogene in the management of tumors, was confirmed as a direct target of miR-138-5p in CRC cells. We also found that the hTERT expression was increased in CRC tissues and was inversely correlated with miR-138-5p. Further study showed that the restoration of hTERT expression by an overexpressing plasmid could reverse the effects of miR-138-5p on proliferation and apoptosis of CRC cells. Taken together, these data defines a major suppresses proliferation and promotes apoptosis role for miR-138-5p, a microRNA functions as a tumor suppressor in CRC, by directly targeting hTERT, which would be provide a new strategy for future CRC therapies.
Keywords: CRC, MicroRNA-138-5p, hTERT, proliferation, apoptosis
Introduction
Colorectal cancer (CRC) is one of the most frequent cancers and leading a significant contributor to cancer death worldwide [1-3]. Despite the availability of treatments such as surgery and chemotherapy for CRC, the survival of this disease remains unsatisfactory [4]. It is an urgent need to look for effective novel anticancer agents for the treatment of human CRC.Thus, increased understanding of molecular mechanism involved in CRC formation and development is important.
MicroRNAs (miRNAs) are a family of small noncoding RNAs that regulate protein coding gene expression by binding to the 3’-untranslated regions (UTRs) of mRNAs [5]. A large body of evidence has indicated that miRNAs modulated multiple important physiological processes such as cancer development [6]. Thus, alterations in miRNA expression are thought to play important roles in cancer initiation and progression through regulating the expression of various oncogenes and tumor suppressors [7-9]. Recent research has showed that miRNAs such as miR-320b, miR-203, miR-451, miR-187, miR-146a, miR-124, miR-101 and miR-34a have been found to regulate the progress in CRC [10-17]. Additionally, miR-138-5p has been found to be regulate tumor progression in a variety of cancers, including larynx carcinoma, bladder cancer, prostate cancer, pancreatic cancer, cervical cancer, thyroid carcinoma and oral squamous cell carcinoma [18-24]. However, the characterization and precise molecular mechanisms of miR-138-5p involved in regulating CRC progression remain unknown.
hTERT is the catalytic subunit of telomerase, which is repressed in normal tissue, but highly expressed in most human tumors and immortal cell lines. It also appears to play animportant role in tumorigenesis [25-27]. miR-138-5p has been reported to be involved in the carcinogenesis and development of various types of cancer by directly targeting hTERT [22,23]. However, whether miR-138-5p function as a tumor suppressor by inhibiting hTERT in human CRC remains unclear.
In the present study, we investigated the potential involvement of miR-138-5p in CRC. We demonstrated that the expression level of miR-138-5p was markedly down-regulated in CRC cell lines and clinical tissues samples. Furthermore, overexpression of miR-138-5p could inhibit proliferation and promote apoptosis in CRC cells. More importantly, we predicted hTERT to be the downstream target of miR-138-5p in CRC cells. Thus, our data suggest that miR-138-5p may be a potential therapeutic target for treating CRC.
Materials and method
Patients and samples
18 paired human CRC tissues and normal corresponding tissues were obtained from archived tissue samples from patients with colorectal cancer who underwent surgical treatment in the Department of General Surgery, Affiliated Hospital of North China University of Science and Technology from July 2013 to August 2015. The specimens were immediately snap-frozen and stored at -80°C in an ultra-low temperature refrigerator until total RNA was extracted. No patient had undergone chemotherapy, radiotherapy and adjuvant treatment before surgery. The study was approved by the Institutional Research Ethics Committee of Affiliated Hospital of North China University of Science and Technology. For the use of these clinical materials for research purposes, informed consent in writing was obtained from each patient.
Cell culture
Five human CRC cell lines (SW620, SW480, LOVO, HCT116, HT29) and a normal colon epithelium cell line (FHC) were purchased from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). 293T cell lines were purchased from the China Cell Culture Center (Shanghai, China). All the cells were cultured in RPMI 1640 (Hyclone, USA) medium, supplemented with 10% fetal bovine serum (FBS, Gibco, USA) and antibiotics (100 U/ml penicillin and 100 ug/ml streptomycin) (Invitrogen, China). The cells were kept in an incubator in a humidified atmosphere with 5% CO2 at 37°C.
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA from tissue samples and cells was extracted using an RNeasy mini kit (Qiagen, Japan) for both miR-138-5p and hTERT mRNA analyses according to the manufacturer’s instructions. RNA quality were measured using the Agilent 2100 Bioanalyzer (Agilent Technologies, USA). cDNA synthesis was performed using PrimeScript RT reagent Kit (Takara, China) according to the manufacturer’s instructions. For detection of miR-138-5p and hTERT mRNA expression, qPCR was performed using the miScriptSYBR® green PCR Kit (Qiagen, USA) according to the manufacturer’s protocol. The relative expression levels of interest gene were calculated by the 2-ΔΔCt method. U6 and β-actin were used as internal controls for miRNAs and mRNAs. the primer for miR-138-5p were: 5’-AGC TGG TGT TGT GAA TCA GGC CG-3’ (sense) and 5’-TGG TGT CGT GGA GTC G-3’ (antisense), and their reverse primer was the universal primer supported by the miScriptSYBR® green PCR Kit (Qiagen, USA). The hTERT mRNA forward primer was 5’-GCA AGT TGC AAA GCA TTG GA-3’ and the reverse primer was 5’-ACC TCT GCT TCC GAC AGC TC-3’. The β-actin primer forward primer was 5’-GGC ACC ACA CCT TCT ACA ATG AG-3’ and the reverse primer was 5’-GGA TAG CAC AGC CTG GAT AGC A-3’.
Cell transfection
miR-138-5p mimic and corresponding mimic negative control (mimic NC), miR-138-5p inhibitor and corresponding inhibitor negative control (inhibitor NC) were designed and brought from GenePharma (Shanghai, China). The fragment of hTERT was amplified by RT-PCR from the tumor tissue and was cloned into eukaryotic expression vector pcDNA3.1, pcDNA3.1-hTERT plasmid was established to make human hTERT overexpression. These molecular productions were transiently transfected into SW480 and HT29 cells using Lipofectamine 2000 Reagent (Invitrogen, USA) according to the manufacturer’s protocol.
Cell proliferation assay
Cell viability was measured using cell countingkit-8 (CCK-8, Dojindo, Japan), according to the manufacturer’s instruction. At 24 h post-transfection with miR-138-5p or mimics NC, SW480 and HT29 cells were seeded onto 96-well plates (2×103 cells/well), and cell proliferation was documented every 24 h for 5 days. The number of viable cells was assessed by measurement of the absorbance at 450 nm using a microplate reader (Tecan, Austria).
Colony formation assay
SW480 and HT29 cells (500 per well) were seeded into a 12-well cell culture plate for 2 weeks. Subsequently, cells were washed twice with PBS, the colonies were stained with 1% crystal violet for 5 min after fixation with 4% paraformaldehyde for 10 min. Colonies were microscopically examined and counted. The assays were repeated 3 times.
Flow cytometry analysis apoptosis
Cell apoptosis was examined by flow cytometry. Briefly, SW480 and HT29 cells were harvested after transfection for 48 h, trypsinized and washed twice with phosphate buffered saline (PBS). Cells (4×105/ml) were collected with l mL PBS and centrifuged at 1000 rpm for 10 min. The cells were suspended in 500 μL of Binding Buffer and detected using an annexin V-fluorescein isothiocyanate (FITC)/PI apoptosis detection kit (BD Biosciences, USA) according to the manufacturer’s protocol under a FACS Calibur flow cytometer (BD Biosciences, USA). The apoptosis ratio was calculated using Cell Quest software (BD Biosciences, USA).
Western blot analysis
The cells in each well were harvested and lysed using a lysis buffer (Beyotime, China) containing proteinase and phosphatase inhibitor cocktails (Sigma-Aldrich, USA). The protein concentrations of the lysates were determined using a bicinchoninic acid protein assay kit (Pierce Biotech, USA). An aliquot of the lysate containing 50 μg proteins was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% nonfat milk for 1 hr and then incubated overnight at 4°C with the following specific primary antibodies. The primary antibodies used include rabbit polyclonal anti-human hTERT (Santa Cruz Biotechnology, USA) and mouse monoclonal anti-human β-actin (Santa Cruz Biotechnology, USA). After three times washing, the membranes were incubated in horseradish peroxidase-conjugated secondary antibody (Amersham Biosciences, USA) for 1 hr at room temperature. Then, the protein band was visualized using the ECL Western blotting substrate (Promega, USA).
Vector construction and luciferase activity assay
For luciferase reporter experiments, luciferase reporter assays were performed in 293T cells. Cells were cotransfected with psiCheck-2 reporter plasmid (Promega, USA) containing the wild type or mutant type of hTERT 3’UTR, along with mimic negative control (NC), miR-138-5p mimic, inhibitor NC and miR-138-5p inhibitor by the Lipofectamine 2000 (Invitrogen, USA). The cells were harvested 24 h after transfection, and luciferase activity was measured with a dual luciferase reporter assay kit (Promega, USA) on a luminometer (Lumat LB9507, Germany).
Statistical analysis
GraphPad Prism 5.0 software and the SPSS 16.0 software was used to conduct all the statistical analyses. The results are represented as mean ± S.D. (standard deviation) of at least three independent experiments. The differences between two experimental conditions were compared on a one-to-one basis using two-tailed Student’s test. Values of P<0.05 was considered to be statistically significant.
Results
miR-138-5p expression is down-regulated in human CRC tissues and cell lines
In order to explore the role of miR-138-5p in human CRC, we detected the expression level of miR-138-5p in 18 paired human CRC tissues and normal corresponding matched tissues using qRT-PCR. miR-138-5p was significantly decreased in CRC tissues compared to that in their matched normal tissues (Figure 1A, P<0.01). Furthermore, we examined the miR-138-5p expression in the normal colon epithelium cell line (FHC) and 5 CRC cell lines, including SW620, SW480, LOVO, HCT116 and HT29. Compared with the FHC, miR-138-5p was also down-regulated in all of the 5 CRC cell lines (Figure 1B, P<0.01). Based on the results, the down-regulated expression of miR-138-5p may participate in the development of CRC.
Figure 1.
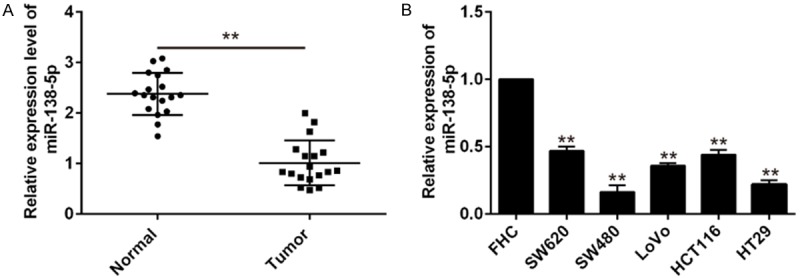
qRT-PCR analysis of miR-138-5p expression in CRC tissue samples and cells. A. Expression of miR-138-5p in 18 pairs of CRC tissue samples and adjacent normal tissues was detected by qRT-PCR, U6 was used as internal control. Data are showed as mean ± S.D. (n = 3). **P<0.01 vs normal. B. The expression levels of miR-138-5p in 5 human CRC cells (SW620, SW480, LOVO, HCT116, HT29) and normal colon epithelium cell line (FHC) were detected by qRT-PCR. Data are showed as mean ± S.D. (n = 3). **P<0.01 vs FHC.
Re-expression of miR-138-5p in CRC cell lines inhibits cell growth, colony formation and enhances apoptosis
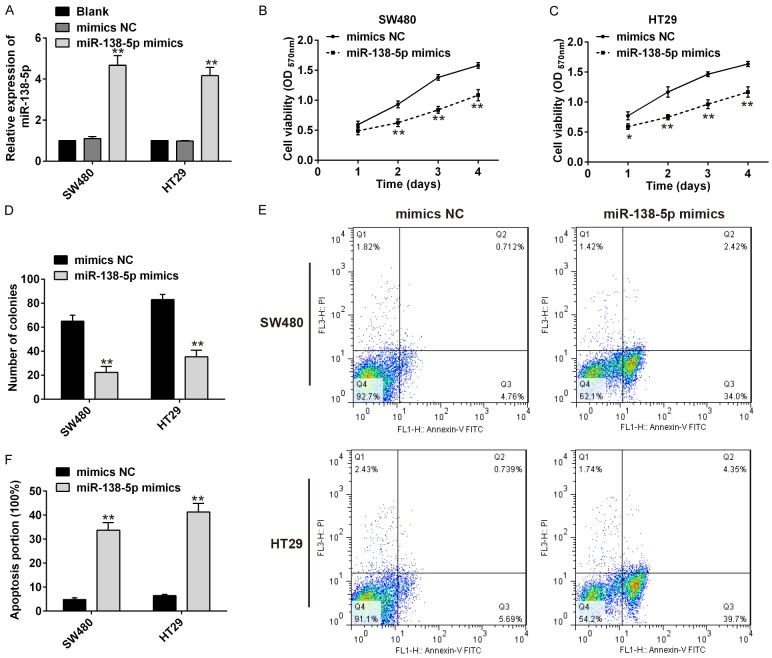
To study the effects of miR-138-5p expression on CRC cells, miR-138-5p mimics or mimics NC was transfected into SW480 and HT29 cells, and qRT-PCR used to confirm up-regulated of miR-138-5p (Figure 2A). The effects of miR-138-5p expression on CRC growth were then examined by a CCK-8 assay and colony formation assays, both SW480 and HT29 cells transfected with miR-138-5p mimics all reduced cell proliferation than the mimics NC group (Figure 2B, 2C). Similarly, colony formation capacity was significantly reduced in SW480 and HT29 cells by miR-138-5p mimics transfection when compared with mimics NC transfection (Figure 2D). In addition, Flow cytometric analysis showed that transfected with miR-138-5p mimics significantly enhanced apoptosis in SW480 and HT29 cells (Figure 2E, 2F).
Figure 2.
Overexpression of miR-138-5p delayed cell proliferation, colony formation and enhances apoptosis. A. QRT-PCR analysis of miR-138-5p in SW480 and HT29 cells transfected with miR-138-5p mimics or mimics NC. **P<0.01 vs blank. B. The CCK8 assay on the proliferation of SW480 cells following transfection with miR-138-5p mimics or mimics NC. C. The CCK8 assay on the proliferation of HT29 cells following transfection with miR-138-5p mimics or mimics NC. D. Colony formation assays of SW480 and HT29 cells transfected with miR-138-5p mimics or mimics NC. E, F. Flow cytometry analysis of apoptosis in miR-138-5p mimics or mimics NC transfected SW480 and HT29 cells. Data are showed as mean ± S.D. (n = 3). **P<0.01 vs mimics NC.
miR-138-5p targets the 3’UTR of hTERT in CRC cells
To explore the underlying mechanism of miR-138-5p in regulating cellular growth and apoptosis in CRC, the predicted target genes of miR-138-5p were screened by TargetScan and RNAhybrid algorithms assay. We found that hTERT target for miR-138-5p since there was a putative miR-138-5p binding sites within the 3’UTR of hTERT mRNA (Figure 3A). To test whether hTERT is a directly target of miR-138-5p, we constructed luciferase reporter plasmids with WT/Mut hTERT 3’UTR. Next, these constructs were transfected into 293T cells with miR-138-5p mimics or miR-138-5p inhibitor. Luciferase activity indicated that transfection of miR-138-5p mimics in the wild-type group efficiently reduced the expression of the luciferase reporter compared with transfection of mimics NC, but transfection of miR-138-5p inhibitor in the wild-type group efficiently increased the expression of the luciferase reporter compared with transfection of inhibitor NC. However, there was no effect was observed in the mut-type group, suggesting that miR-138-5p efficiently controls hTERT expression directly with the 3’UTR of hTERT mRNA (Figure 3B). To validate the miR-138-5p regulation of hTERT in human CRC, SW480 and HT29 cells were transfected with the miR-138-5p mimic or mimics NC. QRT-PCR results showed that upregulation of miR-138-5p expression in SW480 and HT29 cells could decrease hTERT mRNA level (Figure 3C). Western blotting results showed that upregulation of miR-138-5p in SW480 and HT29 cells could also decrease hTERT expression on protein level (Figure 3D). These data showed that miR-138-5p can downregulate hTERT expression by directly targeting its 3’UTR in CRC cells.
Figure 3.
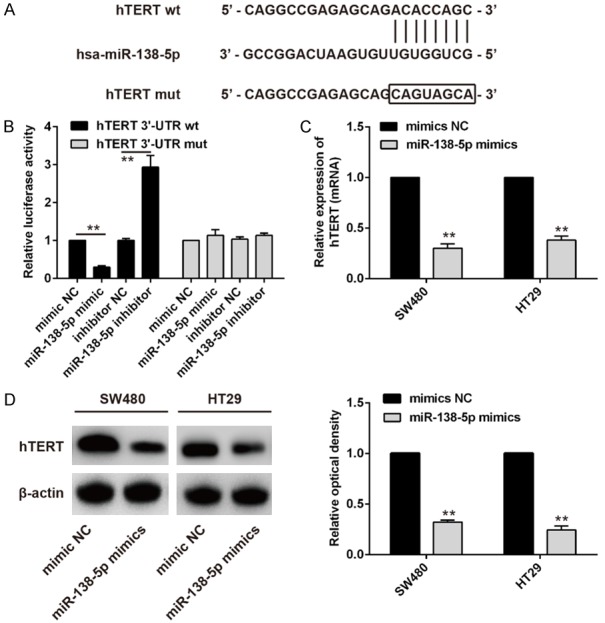
miR-138-5p targets hTERT in human CRC cells. A. The seed regions of the miR-138-5p target sites in hTERT. B. A luciferase reporter plasmid containing wild hTERT 3’UTR or mutant hTERT 3’UTR was transfected into 293T cells alone, cotransfected with mimic NC or miR-138-5p mimic, or with inhibitor NC or miR-138-5p inhibitor and luciferase activity was measured. C. qRT-PCR detection of hTERT mRNA expression in miR-138-5p mimics or mimics NC transfected SW480 and HT29 cells. D. Western blot detection of hTERT protein expression in miR-138-5p mimics or mimics NC transfected SW480 and HT29 cells. Data are showed as mean ± S.D. (n = 3). **P<0.01.
hTERT is up-regulated in CRC tissues and is inversely correlated with miR-138-5p expression
To study the association between miR-138-5p and hTERT, we evaluated the expression of hTERT in human CRC tissues and adjacent normal tissues. Western blotting results showed that hTERT protein level was up-regulated in 7 paired human CRC tissues when compared with corresponding normal tissues (Figure 4A). QRT-PCR results showed that hTERT mRNA expression was also up-regulated in 18 paired human CRC tissues compared to that in their matched normal tissues (Figure 4B). Moreover, we found that hTERT mRNA levels in human CRC tissues were inversely correlated with miR-138-5p expression (Figure 4C, R2 = 0.6356, P<0.01). Taken together, we confirmed that endogenously expressed hTERT is regulated by miR-138-5p expression, miR-138-5p may exert its apoptosis promoting effect through inhibition of hTERT expression.
Figure 4.
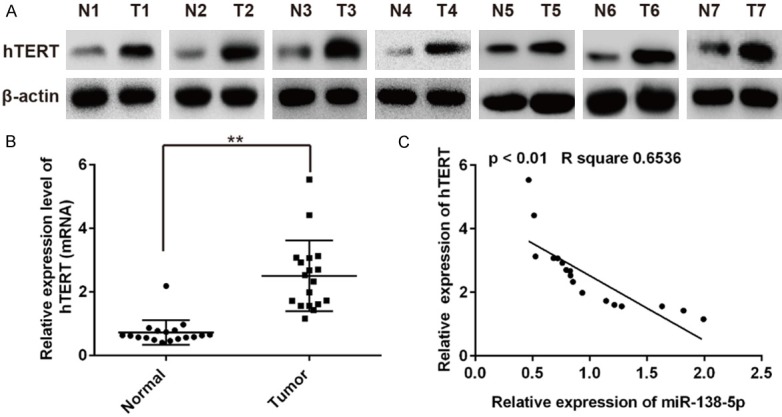
hTERT expression in CRC tissue samples. A. Expression of hTERT in 7 pairs of CRC tissue samples and corresponding adjacent non-tumor tissues were detected by Western blot. B. The mRNA expression level of hTERT in 18 pairs of CRC tissue samples and corresponding adjacent non-tumor tissues. **P<0.01 vs normal. C. miR-138-5p has a reverse correlation with hTERT mRNA expression in 18 paired human CRC tissues and corresponding adjacent non-tumor tissues (R2 = 0.6356, P<0.01). Data are showed as mean ± S.D.
Overexpression of hTERT reverses the anti-tumor effects of the miR-138-5p mimic in human CRC cells
Previous studies have shown miR-138-5p reduced cell proliferation and enhanced cell apoptosis on human CRC cells. Here, we sought to further verify whether these effects were mediated through hTERT. SW480 and HT29 cells were transfected with miR-138-5p mimic for 24 h and followed by transfection with hTERT overexpression vectors. The cell proliferation was determined by a CCK-8 assay. We found that overexpression of hTERT could rescue miR-138-5p mimic induced inhibition of proliferation in SW480 and HT29 cells (Figure 5A). In addition, the cell apoptosis were determined by flow cytometry, overexpression of hTERT could also reverse miR-138-5p mimic induced apoptosis in SW480 and HT29 cells (Figure 5B). These results clearly demonstrated that overexpression of hTERT could at least partially abrogates the anti-tumor effects of miR-138-5p mimic on human CRC cells. Therefore, miR-138-5p may functions as a tumor suppressor gene by directly targeting hTERT on human CRC cells.
Figure 5.
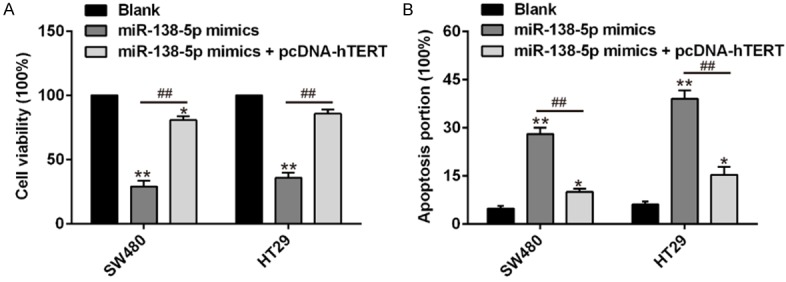
Overexpression of hTERT could partially reverse miR-138-5p mimic induced cell proliferation and apoptosis on human CRC cells. SW480 and HT29 cells were transfected with miR-138-5p mimic or co-transfected with miR-138-5p mimic and overexpression hTERT vectors. A. The cell proliferation was determined in SW480 and HT29 cells by CCK-8 assay. B. The cell apoptosis was determined in SW480 and HT29 cells by flow cytometry. Data are showed as mean ± S.D. (n = 3). *P<0.05, **P<0.01 vs blank; ##P<0.01 vs miR-138-5p mimics.
Discussion
Colorectal cancer is one of the most common cancers, and is a significant contributor to cancer death worldwide [28]. Thus, find out a novel approach to understand the molecular mechanism involved in human CRC initiation and progression is in urgent need. Recently, Growing evidence has demonstrated that dysregulation of miRNAs and hTERT have been suggested to play important roles in the regulation of tumorigenesi in many types of cancer [29-34]. In this study, we identified a crucial tumor suppressive miRNA, miR-138-5p, which plays key roles in the progression in CRC. First, we found that miR-138-5p was down-regulation in CRC tissues and cell lines, up-regulation of miR-138-5p significantly delayed cell proliferation, reduced colony formation and increased apoptosis in CRC cell lines. Secondly, we confirmed that hTERT is a target of miR-138-5p and down-regulated upon miR-138-5p overexpression in CRC cell lines. We also found that the hTERT expression was decreased in CRC tissues and was inversely correlated with miR-138-5p. Finally, hTERT overexpression eliminated the anti-proliferation and pro-apoptotic effects of miR-138-5p in CRC cell lines. Thus, our findings highlight the involvement and crucial role of miR-138-5p in CRC development.
MiRNAs play critical roles in human cancers in which these miRNAs act as either tumor suppressors or promoters and therefore affect tumor development, tumor cell proliferation and apoptosis [35]. Previous studies have indicated that miR-138-5p functioned as tumor suppressor in some cancers [10-17]. For example, Zhao et al. results showed that miR-138-5p as tumor suppressor was down-regulated in CRC tissues and was associated with advanced clinical stage, lymph node metastasis and poor overall survival. It also suppressed CRC cell growth in vitro and inhibited tumorigenesis in vivo by targeting PD-L1 [36]. Gao et al. results showed that the levels of miR-138 was decreased in larynx carcinoma patients specimens, overexpression of miR-138 inhibited ZEB2-mediated larynx carcinoma cell invasiveness [18]. Yu et al. reported that overexpression of miR-138-5p inhibits pancreatic cancer cell growth through targeting FOXC1 [21]. However, the functional role and underlying molecular mechanism of miR-138-5p in CRC remained largely unclear. In the present study, we found that miR-138-5p functions as a tumor suppressor microRNA by directly targeting hTERT in CRC.Functional tests showed that miR-138-5p was frequently down-regulated in human CRC tissues and cell lines, overexpression of miR-138-5p in CRC cells induced cell apoptosis, inhibited cell proliferation and colony formation. It had been reported that miR-138-5p plays the same function in other cancers, our finding showed a consistent with other studies [18,21,36]. In the subsequent mechanism research, hTERT was identified as a target of miR-138-5p, and overexpression of miR-138-5p downregulated hTERT in CRC cells. Further study shows upregulation of hTERT could partially reverse miR-138-5p mimic induced effects in CRC cells. These data suggest that the anti-proliferation and pro-apoptotic role of miR-138-5p may be mediated primarily through hTERT regulation, miR-138-5p would be served as a new therapeutic approach in human CRC.
hTERT is a catalytic subunit of telomerase [37].hTERT have been reported to play a crucial role in the regulation of tumorigenesis and growth in various types of cancer. Down-regulating hTERT expression could obviously suppress the proliferation of bladder cancer, partly change the malignant phenotype and attenuate the tumor growth in xenograft mice model [38]. The expression of hTERT was significantly higher in colorectal laterally spreading tumors tissue samples and cell lines [39]. miR-138-5p has been proven to be involved in the carcinogenesis and development of various types of cancer by directly targeting hTERT. For example, Zhou et al. reported that overexpression of miR-138 inhibited cell proliferation, migration, invasion and tumor growth by targeting hTERT in cervical cancer cells [22]. Shingo et al. reported that miR-138 acts as a tumor suppressor and its downregulation may contribute to the progression through a mechanism involving hTERT in anaplastic thyroid carcinoma cells [23]. Qin et al. reported that miR-138-5p is down-regulated and potentially interact with hTERT in CRC [40]. However, the biological function and regulatory mechanism between miR-138-5p and hTERT in CRC is unknown. In the present study, we demonstrated that targeting hTERT by miR-138-5p was an important regulatory mechanism for cell proliferation and apoptosis in CRC cells, the findings suggest that hTERT might be a common target of miR-138-5p among different types of tumor cells [22,23]. In addition, we confirmed that miR-138-5p downregulated the expression of hTERT in CRC. Overexpression of hTERT could partially reverse miR-138-5p mimic induced effects on human CRC cells. These data further confirmed that hTERT was a potential target of miR-138-5p in human CRC.
In summary, the present study demonstrated that miR-138-5p was significantly down-regulated in CRC tissue samples and cell lines. Moreover, miR-138-5p plays a tumor suppressor role in CRC cells and hTERT is a direct downstream target of miR-138-5p. These findings showed that miR-138-5p may serve as a novel approach for the treatment of patients with CRC in the future.
Disclosure of conflict of interest
None.
References
- 1.Yin Y, Song M, Gu B, Qi X, Hu Y, Feng Y, Liu H, Zhou L, Bian Z, Zhang J, Zuo X, Huang Z. Systematic analysis of key miRNAs and related signaling pathways in colorectal tumorigenesis. Gene. 2016;578:177–184. doi: 10.1016/j.gene.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Konda B, Shum H, Rajdev L. Anti-angiogenic agents in metastatic colorectal cancer. World J Gastrointest Oncol. 2015;7:71–86. doi: 10.4251/wjgo.v7.i7.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Tang S, Le SY, Lu R, Rader JS, Meyers C, Zheng ZM. Aberrant expression of oncogenic and tumor-suppressive microRNAs in cervical cancer is required for cancer cell growth. PLoS One. 2008;3:e2557. doi: 10.1371/journal.pone.0002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebert MS, Sharp PA. Roles for microRNAs in conferring robustness to biological processes. Cell. 2012;149:515–524. doi: 10.1016/j.cell.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esquela-Kerscher A, Slack FJ. OncomirsmicroRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 10.Zhou J, Zhang M, Huang Y, Feng L, Chen H, Hu Y, Chen H, Zhang K, Zheng L, Zheng S. MicroRNA-320b promotes colorectal cancer proliferation and invasion by competing with its homologous microRNA-320a. Cancer Lett. 2015;356:669–675. doi: 10.1016/j.canlet.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong X, Xiao Y, Chen C, Wei X, Hu C, Ling X, Liu X. MicroRNA-203-mediated posttranscriptional deregulation of CPEB4 contributes to colorectal cancer progression. Biochem Biophys Res Commun. 2015;466:206–213. doi: 10.1016/j.bbrc.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Chen MB, Wei MX, Han JY, Wu XY, Li C, Wang J, Shen W, Lu PH. MicroRNA-451 regulates AMPK/mTORC1 signaling and fascin1 expression in HT-29 colorectal cancer. Cell Signal. 2014;26:102–109. doi: 10.1016/j.cellsig.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Sathyanarayanan A, Chandrasekaran KS, Karunagaran D. microRNA-146a inhibits proliferation, migration and invasion of human cervical and colorectal cancer cells. Biochem Biophys Res Commun. 2016;480:528–533. doi: 10.1016/j.bbrc.2016.10.054. [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi K, Sugito N, Kumazaki M, Shinohara H, Yamada N, Nakagawa Y, Ito Y, Otsuki Y, Uno B, Uchiyama K, Akao Y. MicroRNA-124 inhibits cancer cell growth through PTB1/PKM1/PKM2 feedback cascade in colorectal cancer. Cancer Lett. 2015;363:17–27. doi: 10.1016/j.canlet.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 15.Chen MB, Yang L, Lu PH, Fu XL, Zhang Y, Zhu YQ, Tian Y. MicroRNA-101 down-regulates sphingosine kinase 1 in colorectal cancer cells. Biochem Biophys Res Commun. 2015;463:954–960. doi: 10.1016/j.bbrc.2015.06.041. [DOI] [PubMed] [Google Scholar]
- 16.Lu G, Sun Y, An S, Xin S, Ren X, Zhang D, Wu P, Liao W, Ding Y, Liang L. MicroRNA-34a targets FMNL2 and E2F5 and suppresses the progression of colorectal cancer. Exp Mol Pathol. 2015;99:173–179. doi: 10.1016/j.yexmp.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Zhang F, Luo Y, Shao Z, Xu L, Liu X, Niu Y, Shi J, Sun X, Liu Y, Ding Y, Zhao L. MicroRNA-187, a downstream effector of TGFbeta pathway, suppresses Smad-mediated epithelial-mesenchymal transition in colorectal cancer. Cancer Lett. 2016;373:203–213. doi: 10.1016/j.canlet.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 18.Gao S, Wang J, Xie J, Zhang T, Dong P. Role of miR-138 in the regulation of larynx carcinoma cell metastases. Tumour Biol. 2015 doi: 10.1007/s13277-015-4244-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Yang R, Liu M, Liang H, Guo S, Guo X, Yuan M, Lian H, Yan X, Zhang S, Chen X, Fang F, Guo H, Zhang C. miR-138-5p contributes to cell proliferation and invasion by targeting survivin in bladder cancer cells. Mol Cancer. 2016;15:82. doi: 10.1186/s12943-016-0569-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erdmann K, Kaulke K, Rieger C, Salomo K, Wirth MP, Fuessel S. MiR-26a and miR-138 block the G1/S transition by targeting the cell cycle regulating network in prostate cancer cells. J Cancer Res Clin Oncol. 2016;142:2249–2261. doi: 10.1007/s00432-016-2222-4. [DOI] [PubMed] [Google Scholar]
- 21.Yu C, Wang M, Li Z, Xiao J, Peng F, Guo X, Deng Y, Jiang J, Sun C. MicroRNA-138-5p regulates pancreatic cancer cell growth through targeting FOXC1. Cell Oncol (Dordr) 2015;38:173–181. doi: 10.1007/s13402-014-0200-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou N, Fei D, Zong S, Zhang M, Yue Y. MicroRNA-138 inhibits proliferation, migration and invasion through targeting hTERT in cervical cancer. Oncol Lett. 2016;12:3633–3639. doi: 10.3892/ol.2016.5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitomo S, Maesawa C, Ogasawara S, Iwaya T, Shibazaki M, Yashima-Abo A, Kotani K, Oikawa H, Sakurai E, Izutsu N, Kato K, Komatsu H, Ikeda K, Wakabayashi G, Masuda T. Downregulation of miR-138 is associated with overexpression of human telomerase reverse transcriptase protein in human anaplastic thyroid carcinoma cell lines. Cancer Sci. 2008;99:280–286. doi: 10.1111/j.1349-7006.2007.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manikandan M, Deva Magendhra Rao AK, Rajkumar KS, Rajaraman R, Munirajan AK. Altered levels of miR-21, miR-125b-2*, miR-138, miR-155, miR-184, and miR-205 in oral squamous cell carcinoma and association with clinicopathological characteristics. J Oral Pathol Med. 2015;44:792–800. doi: 10.1111/jop.12300. [DOI] [PubMed] [Google Scholar]
- 25.Noel JF, Wellinger RJ. Exposing secrets of telomere-telomerase encounters. Cell. 2012;150:453–454. doi: 10.1016/j.cell.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Greider CW, Blackburn EH. Telomeres, telomerase and cancer. Sci Am. 1996;274:92–97. doi: 10.1038/scientificamerican0296-92. [DOI] [PubMed] [Google Scholar]
- 27.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 28.Lambert R, Sauvaget C, Sankaranarayanan R. Mass screening for colorectal cancer is not justified in most developing countries. Int J Cancer. 2009;125:253–256. doi: 10.1002/ijc.24371. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y, Yang JJ, Tao H, Jin WS. MicroRNA-21 controls hTERT via PTEN in human colorectal cancer cell proliferation. J Physiol Biochem. 2015;71:59–68. doi: 10.1007/s13105-015-0380-5. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Q, Zhai YX, Liu HQ, Shi YA, Li XB. MicroRNA-491-5p suppresses cervical cancer cell growth by targeting hTERT. Oncol Rep. 2015;34:979–986. doi: 10.3892/or.2015.4013. [DOI] [PubMed] [Google Scholar]
- 31.Chen L, Lu MH, Zhang D, Hao NB, Fan YH, Wu YY, Wang SM, Xie R, Fang DC, Zhang H, Hu CJ, Yang SM. miR-1207-5p and miR-1266 suppress gastric cancer growth and invasion by targeting telomerase reverse transcriptase. Cell Death Dis. 2014;5:e1034. doi: 10.1038/cddis.2013.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Lei H, Xu Y, Tao ZZ. miR-512-5p suppresses tumor growth by targeting hTERT in telomerase positive head and neck squamous cell carcinoma in vitro and in vivo. PLoS One. 2015;10:e0135265. doi: 10.1371/journal.pone.0135265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z, Liu YH, Diao HY, Ma J, Yao YL. MiR-661 inhibits glioma cell proliferation, migration and invasion by targeting hTERT. Biochem Biophys Res Commun. 2015;468:870–876. doi: 10.1016/j.bbrc.2015.11.046. [DOI] [PubMed] [Google Scholar]
- 34.Zhang D, Xiao YF, Zhang JW, Xie R, Hu CJ, Tang B, Wang SM, Wu YY, Hao NB, Yang SM. miR-1182 attenuates gastric cancer proliferation and metastasis by targeting the open reading frame of hTERT. Cancer Lett. 2015;360:151–159. doi: 10.1016/j.canlet.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 35.Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell. 2012;148:1172–1187. doi: 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao L, Yu H, Yi S, Peng X, Su P, Xiao Z, Liu R, Tang A, Li X, Liu F, Shen S. The tumor suppressor miR-138-5p targets PD-L1 in colorectal cancer. Oncotarget. 2016;7:45370–45384. doi: 10.18632/oncotarget.9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cong YS, Wright WE, Shay JW. Human telomerase and its regulation. Microbiol Mol Biol Rev. 2002;66:407–425. doi: 10.1128/MMBR.66.3.407-425.2002. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zou L, Zhang P, Luo C, Tu Z. shRNA-targeted hTERT suppress cell proliferation of bladder cancer by inhibiting telomerase activity. Cancer Chemother Pharmacol. 2006;57:328–334. doi: 10.1007/s00280-005-0056-x. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Cao X, Fang Y, Liao ZE, Liu YY, Huang BD, Han YJ. Overexpression of hTERT in potentially malignant colorectal laterally spreading tumors. Mol Med Rep. 2013;7:1409–1412. doi: 10.3892/mmr.2013.1376. [DOI] [PubMed] [Google Scholar]
- 40.Qin YZ, Xie XC, Liu HZ, Lai H, Qiu H, Ge LY. Screening and preliminary validation of miRNAs with the regulation of hTERT in colorectal cancer. Oncol Rep. 2015;33:2728–2736. doi: 10.3892/or.2015.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]