Abstract
Childhood Guillain-Barré syndrome (GBS) occasionally leads to respiratory failure early after onset, requiring long-term ventilation management after tracheal intubation. However, patients requiring tracheostomy management are rare. In the present study, a case of a 12-year-old boy with GBS who required artificial respiration management due to rapid progression of respiratory muscle paralysis is reported. Intravenous immunoglobulin (IVIg) and pulse steroid therapy were provided; however, both were ineffective and tracheostomy was necessary 26 days after onset. A second course of IVIg and pulse steroid therapy was administered on day 34. With continued rehabilitation, the patient was able to walk long distances on day 74 and was subsequently discharged on day 89. In cases of severe GBS, when IVIg and pulse steroid therapy do not improve the respiratory muscle strength of the patient, early tracheostomy may improve the patient's quality of life during artificial respiration management.
Keywords: Guillain-Barré syndrome, children, tracheostomy, immunoglobulin, steroid pulse
Introduction
Guillain-Barré syndrome (GBS) is an acute motor-neuron, peripheral neuropathy characterized by symmetrical ascending motor paralysis and a generally monophasic clinical course. The clinical manifestations of GBS include attenuation or disappearance of tendon reflexes, sensory disorders, such as numbness and pain, autonomic nervous system disorders, such as bradycardia and tachycardia, arrhythmias, abnormal blood pressure, and intestinal motility abnormalities. Moreover, 20–30% of patients require mechanical ventilation because of respiratory failure, and approximately half of the patients have cranial nerve deficits, primarily facial palsy (1). Generally, paralysis symptoms appear 1–2 weeks after infection, the symptoms peak within 4 weeks, and then, the patients recover (2,3). The most common treatments for GBS are a combination of methylprednisolone steroid pulse therapy and intravenous immunoglobulin (IVIg), and a current double-blind, randomized clinical trial is on-going to assess the effect of a second IVIg course in severely affected GBS patients (4). Occasionally, however, patients may require long-term artificial respiratory management secondary to respiratory muscle paralysis (5,6). In children, the incidence of mechanical ventilation is quite high, with reported rates of 19.2% (7), 20% (8) and 14% (9). Although tracheostomy may improve the patient's quality of life during long-term ventilation management, this approach has been infrequent among children with GBS.
In the present study, the clinical course of a patient with GBS who required artificial respiration management and tracheostomy due to rapid progression of respiratory muscle paralysis is reported.
Case report
The study was approved by the Ethics Committee of the Dokkyo Medical University (Mibu, Japan). Formal written informed consent was obtained from the parents of the patient. Our patient, recruited on July 2015, was a healthy 12-year-old boy with a history of febrile convulsions at 4 months of age. He reported allergies to mackerel and buckwheat, and had an unremarkable family medical history. Prior to visiting the hospital, he developed common cold symptoms accompanied by fever and headache, which improved in a few days. However, during that 1st week, he developed numbness in his fingers and limbs prompting a visit to the Neurosurgery Department on day 8. Head computed tomography revealed normal findings. On day 9, he visited a private clinic because of vomiting, received intravenous fluid therapy without improvement, and was subsequently admitted to a general hospital. Although he was able to sit up at the time of hospital admission, he found it difficult to walk unassisted for long periods. Magnetic resonance imaging of his head and spinal cord showed no abnormal findings. On day 12, he was urgently transferred to our University Hospital because of dysphagia and dyspnea.
On admission, he weighed 51 kg, had a height of 155 cm, body temperature of 36.6°C, heart rate >100/min, and blood pressure of 168/116 mmHg. His oxygen saturation was 92% breathing room air and his consciousness level remained high. He produced weak respiratory sounds without a heart murmur, and attenuated intestinal peristalsis on chest and abdominal auscultation. The patient had difficulty keeping his eyes closed which were noticed to be dry. Neurological examination revealed a decreased muscle tone in his limbs, with manual muscle testing (MMT) showing bilateral symmetry, upper limb grades of 2, lower limb grades of 0–1, and lower-limb-dominant muscle weakness. Numbness and a decline in the superficial sensation of his hands and fingers with absent deep tendon reflexes in both upper and lower limbs were also observed.
Blood examination showed elevated white blood cell count (15,700/µl) and platelet count (60×104/µl). Additionally, the patient tested negative for Epstein-Barr virus, cytomegalovirus, herpes simplex virus, varicella-zoster virus, and Mycoplasma spp. infections. Stool cultures detected resident bacteria, and were negative for Campylobacter spp. Levels of anti-ganglioside antibodies, including GM1, GM1b, GD1a, Ga1NAc-GD1a, GQ1b, GD1b and GT1a, were not markedly increased. Peripheral nerve conduction velocity testing revealed decreased median nerve action potential, prolonged distal latency of midline and peroneal nerves, and absent F-waves in midline and tibial nerves. Accordingly, based on electrophysiological testing, the patient was diagnosed as having a demyelinating type of childhood GBS.
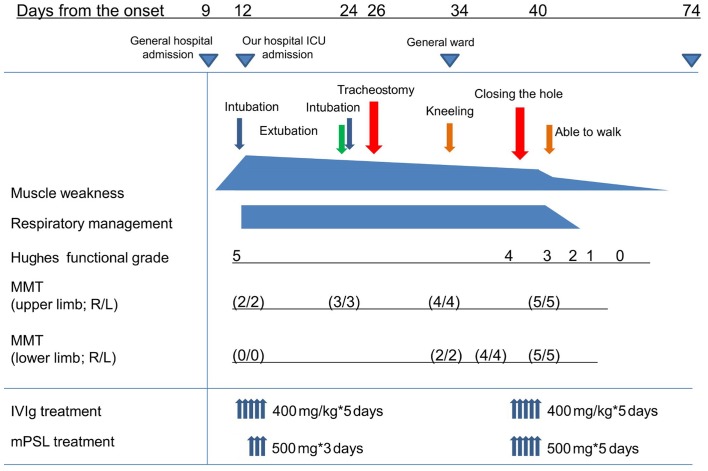
The patient's clinical course after hospitalization is presented in Fig. 1. Hughes functional grade score was 4 (difficulty walking 5 m even with support) on admission. However, the patient's breathing difficulty progressed rapidly, and 3 h after admission, tracheal intubation was performed which increased his functional grade to 5 (requiring supplemental ventilation). The patient was transferred to an Intensive Care Unit, and immediate IVIg therapy (400 mg/kg × 5 days) and pulse methylprednisolone therapy (500 mg × 3 days) were prescribed. Ventilator withdrawal and extubation were attempted on day 24 after some improvement in his neck and upper limb strength (MMT score: 3). However, his breathing difficulty worsened, and a second tracheal intubation was necessary after 3 h. On day 26, it was clear that long-term intubation management would be required. After obtaining written informed consent from the parents of the patient, an otolaryngologist performed a tracheostomy. Before the tracheostomy, the patient had been dependent on a ventilator for respiration; however, his respiratory condition improved gradually and he was transferred from the Intensive Care Unit to the general ward a few days later. A second course of IVIg and pulse corticosteroid therapy were performed on day 34. On day 40, the patient's breathing improved and his tracheostomy was closed when he no longer required mechanical ventilation. Joint movement remained difficult because of intense nerve root pain in his ankle; however, the pain disappeared after the second course of IVIg and pulse steroid therapy. After continued rehabilitation, he was able to walk long distances (functional grade: 3, 2 and 0 on days 56, 66 and 74, respectively), and he was subsequently discharged on day 89.
Figure 1.
Clinical course of patient with GBS. GBS, Guillain-Barré syndrome; MMT, manual muscle testing; IVIg, intravenous immunoglobulin; mPSL, methylpredonisolone pulse.
Discussion
Some studies have presented the need for artificial ventilation management because of rapid muscle weakness in patients with GBS (10,11), and childhood-onset GBS poses a higher risk of requiring artificial respiration management (12).
Wijdicks (13) has reported that tracheostomy is strongly recommended after 2 weeks to avoid tracheomalacia and postintubation stenosis. Principi et al (14) have reported that elective tracheostomy is rarely performed among children in Canada; however, 51% of the physicians surveyed consider that it is underutilized. In addition, Walgaard et al (15) have developed a prediction tool for the selection of GBS patients, among patients receiving mechanical ventilation, for early tracheostomy. Thus, physicians should be aware of the indications for both artificial respiratory management and tracheostomy in patients with GBS, especially in children. Table I presents details on 7 Japanese children patients with GBS who required ventilator management (16–23). As shown in the Table, the children who ranged in age from 2–12 years, had early clinical features, such as dysphonia, difficulty in expectoration, and dysphagia, suggesting a decrease in respiratory muscle strength. Table I also shows the number of intubation days required; however, no patients underwent tracheostomy for artificial respiration management. Moreover, in a clinical study of 91 childhood GBS cases in Japan, 19 patients (21.6%) required artificial ventilation management, and none received tracheostomy (20). Another report has shown that among 50 adult patients with GBS, 15 underwent tracheostomy, subsequently resulting in a good clinical course and improved quality of life (5). For the patient of the present study, IVIg and pulse steroid therapy did not improve respiration and whole-body muscle strength. Given that the attempt of ventilator withdrawal and extubation led to worsened symptoms, it was decided to perform tracheostomy early during the patient's clinical course, and it was possible to surgically close the tracheostomy soon afterwards, because of the good clinical course that followed. Childhood GBS may occasionally lead to sudden respiratory failure requiring long-term respiratory management. In severe cases, in which the respiratory muscle strength does not improve with IVIg and pulse steroid therapy, early tracheostomy should be considered, as it may improve the patient's quality of life during prolonged artificial respiration management.
Table I.
Mechanical ventilation management in Japanese pediatric patients with GBS from 2000 to 2015.
| Studies | Age (years) | Sex | Characteristic initial symptoms | Intubation from admission (days) | Intubation management (days) | IVIg treatment | mPSL treatment | Outcomes | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| Kawai et al | 2 | Male | Expectoration difficulty, hoarseness | Nd | 8 | + | Nd | Extubation and discharge | (16) |
| Minamihara et al | 3 | Female | Swallowing difficulty, hoarseness | 2 | 18 | + | + | Extubation and discharge on day 58 | (17) |
| Takeshita et al | 4 | Male | Expectoration difficulty, dysphonia | Nd | 16 | + | Nd | Extubation and discharge on day 75 | (18) |
| Shibata et al | 8 | Male | Breathing difficulty | 2 | 5 | + | Nd | Extubation and discharge | (19) |
| Arashin et al | 10 | Female | Shallow breathing | 2 | Nd | Nd | Nd | Extubation and discharge | (20) |
| Yata et al | 10 | Male | Diplopia, gait disturbance | Nd | Nd | + | Nd | Immunosorbent therapy, extubation, and discharge | (21) |
| Kawai and Shimizu | 11 | Female | Bulbar palsy | Nd | Nd | + | Nd | Adenovirus infection, extubation, and discharge | (22) |
| Present study | 12 | Male | Expectoration difficulty, breathing difficulty | 4 | 15 | + | + | 15 days after tracheotomy, breathing had improved and the hole was closed |
GBS, Guillain-Barré syndrome; IVIg, intravenous immunoglobulin; mPSL, methylprednisolone pulse; Nd, not described.
Acknowledgements
We would like to thank Dr Yao Lu (Seiko Sciences) and Jane Charbonneau, DVM, from Edanz Group, for editing a draft of this manuscript.
Glossary
Abbreviations
- GBS
Guillain-Barré syndrome
- IVIg
intravenous immunoglobulin
- MMT
manual muscle testing
Funding
No funding was received.
Availability of data and materials
The data used and/or analyzed during the current study is available from the corresponding author on reasonable request.
Authors' contributions
MM, GIm, GIc and SY were involved in the writing of the manuscript. MM and GIc were the patient's doctors. YS, YK and KW were involved in the patient's intensive care treatment and collected the blood test results. TK operated on the patient and planned the patient's clinical course. KF, TN and NK analyzed the biological data of the patient and evaluated the results of the physiological peripheral nerve testing. GIm was responsible for monitoring the patient's neurological status in Intensive Care Unit. MM and SY were responsible for the medical strategy for patient's respiratory failure. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Dokkyo Medical University. Formal written informed consent was obtained from the parents of the patient.
Patient consent for publication
The authors received formal written informed consent from the parents of the patient approving the publication of these data.
Competing interests
The authors declare that they have no competing interests.
References
- 1.van den Berg B, Walgaard C, Drenthen J, Fokke C, Jacobs BC, van Doorn PA. Guillain-Barré syndrome: Pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. 2014;10:469–482. doi: 10.1038/nrneurol.2014.121. [DOI] [PubMed] [Google Scholar]
- 2.Hughes RA, Cornblath DR. Guillain-Barré syndrome. Lancet. 2005;366:1653–1666. doi: 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- 3.Yuki N, Hartung HP. Guillain-Barré syndrome. N Engl J Med. 2012;366:2294–2304. doi: 10.1056/NEJMra1114525. [DOI] [PubMed] [Google Scholar]
- 4.Walgaard C, Jacobs BC, Lingsma HF, Steyerberg EW, Cornblath DR, van Doorn PA, van Doorn PA, Jacobs BC, Walgaard C, de Wit MC, et al. Dutch GBS Study Group Second IVIg course in Guillain-Barré syndrome patients with poor prognosis (SID-GBS trial): Protocol for a double-blind randomized, placebo-controlled clinical trial. J Peripher Nerv Syst. 2018;23:210–215. doi: 10.1111/jns.12286. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida M, Ikeda J, Urikane Y, Kashiwada T, Kaseda Y, Kohriyama T. Prevalence of tracheotomy and percutaneous endoscopic gastrostomy in patients with Guillain-Barré syndrome. Dysphagia. 2017;32:236–240. doi: 10.1007/s00455-016-9750-6. [DOI] [PubMed] [Google Scholar]
- 6.Fletcher DD, Lawn ND, Wolter TD, Wijdicks EF. Long-term outcome in patients with Guillain-Barré syndrome requiring mechanical ventilation. Neurology. 2000;54:2311–2315. doi: 10.1212/WNL.54.12.2311. [DOI] [PubMed] [Google Scholar]
- 7.Kalra V, Sankhyan N, Sharma S, Gulati S, Choudhry R, Dhawan B. Outcome in childhood Guillain-Barré syndrome. Indian J Pediatr. 2009;76:795–799. doi: 10.1007/s12098-009-0125-y. [DOI] [PubMed] [Google Scholar]
- 8.Kalita J, Kumar M, Misra UK. Prospective comparison of acute motor axonal neuropathy and acute inflammatory demyelinating polyradiculoneuropathy in 140 children with Guillain-Barré syndrome in India. Muscle Nerve. 2018;57:761–765. doi: 10.1002/mus.25992. [DOI] [PubMed] [Google Scholar]
- 9.Roodbol J, de Wit MC, Aarsen FK, Catsman-Berrevoets CE, Jacobs BC. Long-term outcome of Guillain-Barré syndrome in children. J Peripher Nerv Syst. 2014;19:121–126. doi: 10.1111/jns5.12068. [DOI] [PubMed] [Google Scholar]
- 10.Sundar U, Abraham E, Gharat A, Yeolekar ME, Trivedi T, Dwivedi N. Neuromuscular respiratory failure in Guillain-Barré syndrome: Evaluation of clinical and electrodiagnostic predictors. J Assoc Physicians India. 2005;53:764–768. [PubMed] [Google Scholar]
- 11.El-Bayoumi MA, El-Refaey AM, Abdelkader AM, El-Assmy MM, Alwakeel AA, El-Tahan HM. Comparison of intravenous immunoglobulin and plasma exchange in treatment of mechanically ventilated children with Guillain Barré syndrome: A randomized study. Crit Care. 2011;15:R164. doi: 10.1186/cc10305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu X, Shen D, Li T, Zhang B, Li C, Mao M, Zhao J, Liu K, Zhang HL. Distinct clinical characteristics of pediatric Guillain-Barré syndrome: A comparative study between children and adults in Northeast China. PLoS One. 2016;11:e0151611. doi: 10.1371/journal.pone.0151611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wijdicks EF. The neurology of acutely failing respiratory mechanics. Ann Neurol. 2017;81:485–494. doi: 10.1002/ana.24908. [DOI] [PubMed] [Google Scholar]
- 14.Principi T, Morrison GC, Matsui DM, Speechley KN, Seabrook JA, Singh RN, Kornecki A. Elective tracheostomy in mechanically ventilated children in Canada. Intensive Care Med. 2008;34:1498–1502. doi: 10.1007/s00134-008-1104-x. [DOI] [PubMed] [Google Scholar]
- 15.Walgaard C, Lingsma HF, van Doorn PA, van der Jagt M, Steyerberg EW, Jacobs BC. Tracheostomy or not: Prediction of prolonged mechanical ventilation in Guillain-Barré syndrome. Neurocrit Care. 2017;26:6–13. doi: 10.1007/s12028-016-0311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawai H, Shikano H, Honbu K, Ito K, Iwata A, Fujii H, Nakajima Y. A child with Guillain-Barré syndrome who needed artificial ventilation management. Jpn J Pediatr Soc. 2015;119:904. (In Japanese) [Google Scholar]
- 17.Minamihara T, Okizuka Y, Kameoka K, Kitahara H, Nakata A, Gouma T, Ueda Y, Hashimura Y, Hayashi S, Minami H. A case of Guillain-Barré syndrome who presented with hoarseness as a chief complaint. Jpn J Pediatr Soc. 2015;119:631. (In Japanese) [Google Scholar]
- 18.Takeshita K, Takagi A, Mizuoti H, Uchikawa H, Misawa S, Ikeda K, Fujii K. A case of childhood fulminant Guillain-Barré syndrome requiring artificial respiration management on day 2 from admission. No To Hattatsu. 2014;46:S340. (In Japanese) [Google Scholar]
- 19.Shibata T, Oka M, Kobayashi K, Yoshinaga H. A case of axonal Guillain-Barré syndrome with acute respiratory failure. Jpn J Pediatr Soc. 2014;118:844. (In Japanese) [Google Scholar]
- 20.Arashin O, Kitaura N, Shimooka T, Furukawa I, Shiote Y, Wagou M. An infantile case of Guillain-Barré syndrome requiring artificial respiratory management. J Hiroshima Med Assoc. 2013;66:464. (In Japanese) [Google Scholar]
- 21.Yata Y, Hara S, Matsunaga Y, Tada H, Shimizu S, Tagaya M, Fujimaki H, Kido S, Okumura N, Okada J. A case of Guillain-Barré syndrome with secondary adenovirus infection. Jpn J Pediatr Soc. 2012;16:1393. (In Japanese) [Google Scholar]
- 22.Kawai N, Shimizu Y. Immunoabsorption plasma pheresis for Guillain Barré syndrome in children: A review. Med J Nishio Munic Hosp. 2004;15:14–16. (In Japanese) [Google Scholar]
- 23.Hirano Y, Osawa M, Ooya T, Sakakibara Y, Nihei K, Maekawa K, Momoi M, Yata J. Guillain-Barré syndrome in children - Questionnaire survey on prevalence, therapies, and clinical outcomes. Jpn J Pediatr. 2000;53:77–86. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used and/or analyzed during the current study is available from the corresponding author on reasonable request.