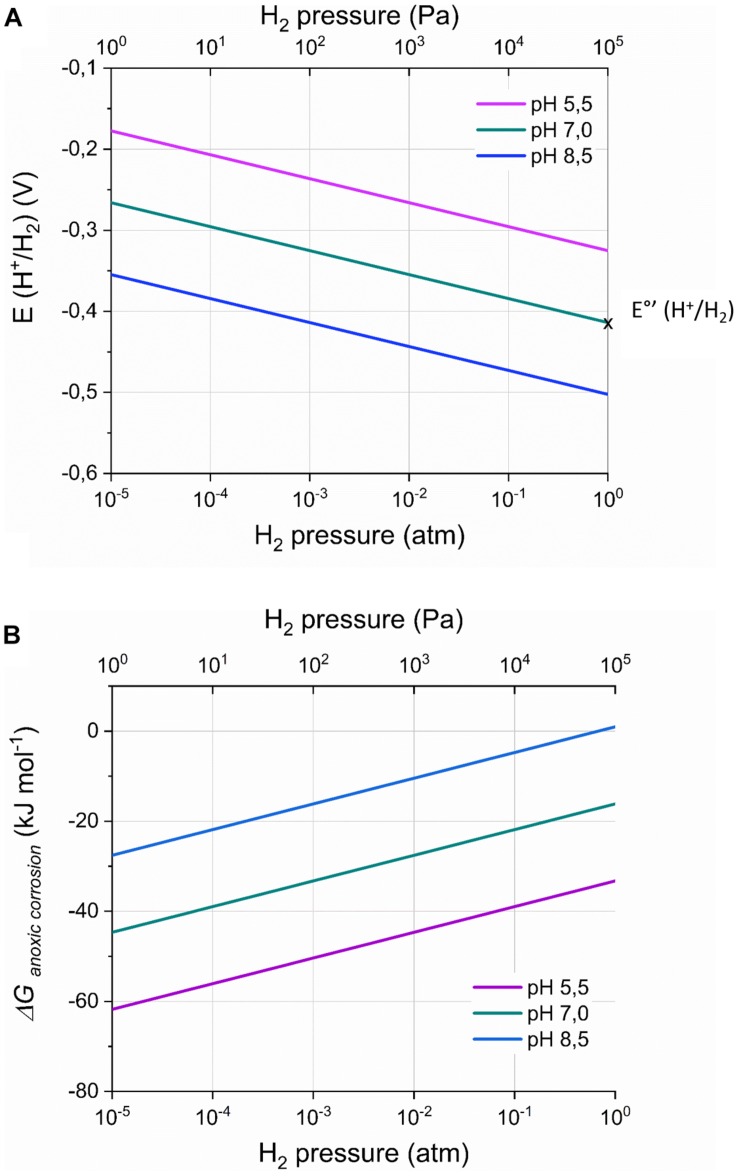
FIGURE 2.
The onset potential for H2 evolution by a cathode E(H+/H2) (V) (A) and the Gibbs free energy change (ΔGanoxic corrosion; kJ mol– 1) of the anoxic corrosion reaction (B) in function of the H2 partial pressure and the pH, according to respectively Equations 1 and 3. The standard H2 potential at pH 7 [E°’(H+/H2)] is indicated on the graph. ΔGanoxic corrosion was calculated assuming a Fe2+ concentration of 1 mM. Remark the logarithmic scale for the H2 partial pressures.