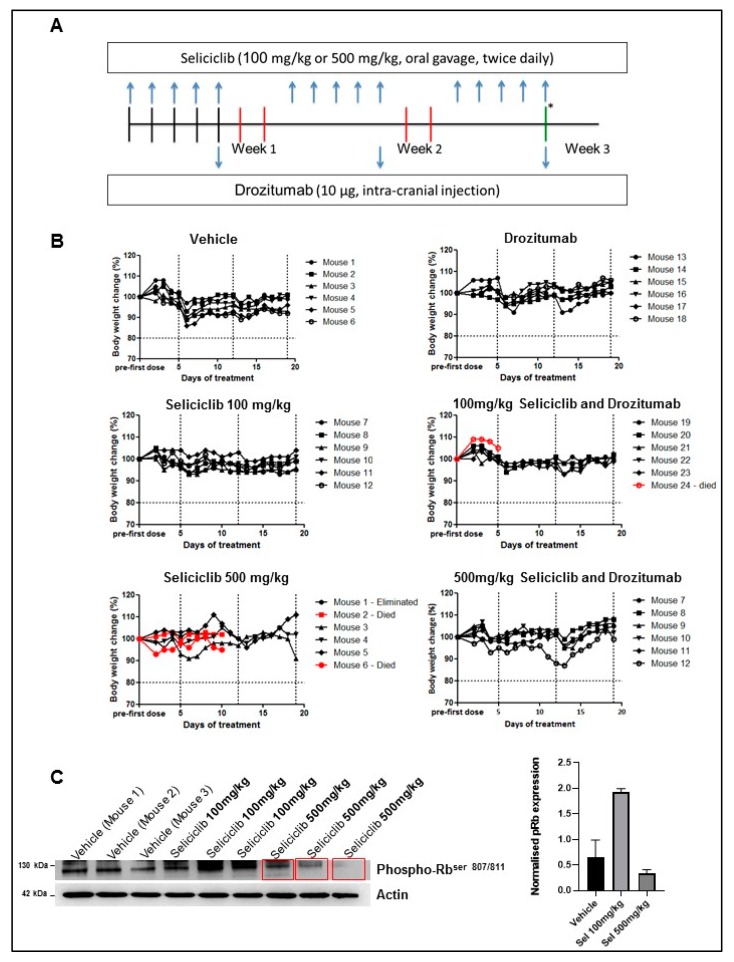
Figure 2.
In vivo toxicity findings associated with first-generation CDK inhibitor, seliciclib, in combination with the antibody against human death receptor 5, drozitumab combined treatment. (A) Mice were treated as indicated in the toxicity study workflow. Animals were monitored daily and scored for changes in weight loss and behavior as signs of toxic effect. After three weeks, mice were sacrificed by cervical dislocation. (B) Body weights of animals that were treated with: (1) vehicles for both routes of drug administration (10% dimethyl sulfoxide (DMSO):5% Tween 20:85% 50 mM hydrochloric acid (HCl)/saline) by oral gavage, twice daily, Monday–Friday, and sterile H2O by intracranial injection, once weekly, for three weeks; (2) drozitumab, 10 μg intra-cranially once weekly for three weeks; (3) 100 or 500 mg/kg seliciclib, delivered by oral gavage twice daily, Monday–Friday for three weeks; (4) A combination of seliciclib (100 or 500 mg/kg) plus drozitumab over a three-week period (n = 6 per group). (C) Western Blot analysis of isolated brain tissue confirmed that the animals that received the higher dose of seliciclib, 500 mg/kg, had a definite and reproducible down-regulation of phospho-Rb. Actin was used as a loading control. The uncropped blots and molecular weight markers are shown in supplementary materials.