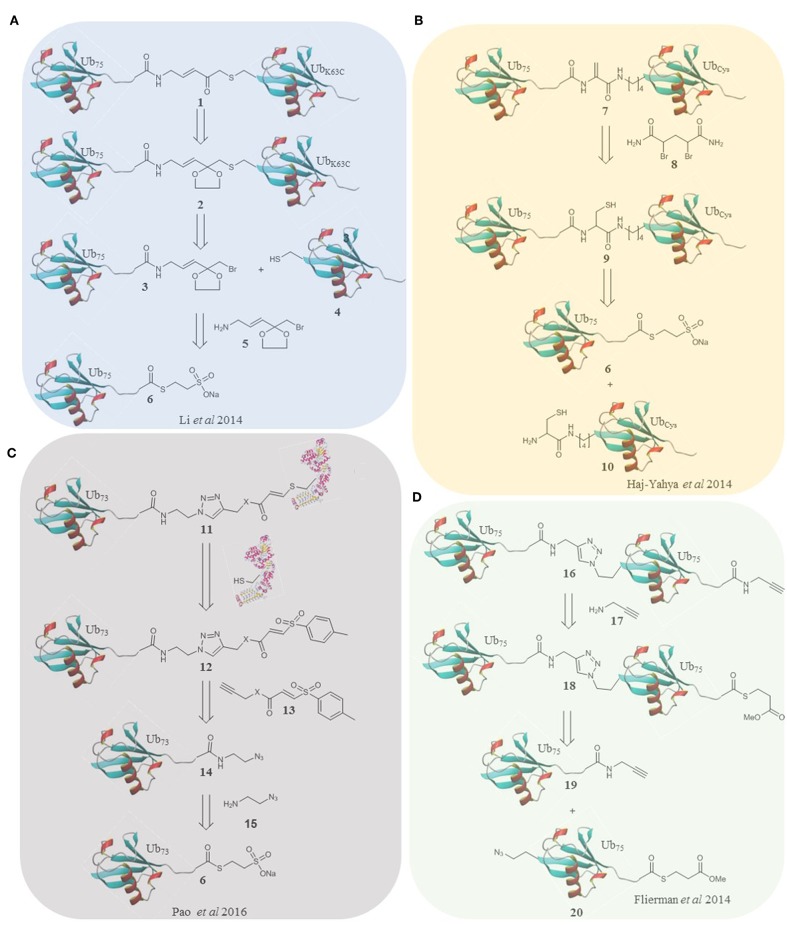
Figure 1.
Retrosynthesis of selected probes. (A) A methodology reported by Li et al. utilizes an α-bromo-vinylketal to link Ub75 thioester 6 and the mutated Ub monomer 4. Deprotection of the ketal of 2 unmasks a Michael acceptor within the linker of the probe 1. (B) Haj-Yahya et al. synthesized a diubiquitin probe based on DHA as the electrophilic warhead. NCL is used to link Ub monomers 6 and 10, positioning a cysteine residue at position 76 of the distal Ub which is then converted to DHA using the dibromide reagent 7. (C) Pao et al. expanded on the TDAE methodology to incorporate a Ub monomer and E2 enzyme in a single probe. Alkyne functionalized TDAE 13 is coupled to azido functionalized Ub monomer 14 using copper catalyzed cycloaddition. A subsequent reaction with an E2 enzyme eliminates the tosyl component of the TDAE 12, affording the final probe 11 containing a Michael acceptor. (D) Flieman et al. use copper catalyzed cycloaddition to conjugate two modified Ub monomers 19 and 20. Propargyl amine 17 was reacted with the C-terminus of the proximal monomer to yield probe 16.