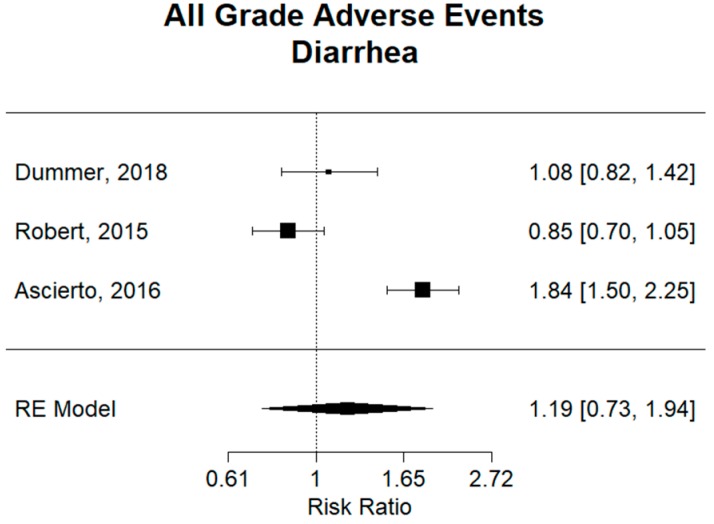
Figure 7.
Relative risk (RR) of “diarrhea” adverse events; all grades. Combined RR for all studies was 1.19 (95% CI 0.73–1.94), showing no significant difference in risk of diarrhea between combination and monotherapy groups. There is no significant difference in encorafenib plus binimetinib in the trial by Dummer (RR: 1.08, 95% CI 0.82–1.42) or dabrafenib plus trametinib in the trial by Robert (RR: 0.85, 95% CI 0.70–1.05) when compared to vemurafenib monotherapy. Vemurafenib plus cobimetinib in the trial by Ascierto had a relative risk of 1.84 (95% CI 1.50–2.25), showing a statistically significant high risk of diarrhea when compared to vemurafenib monotherapy.