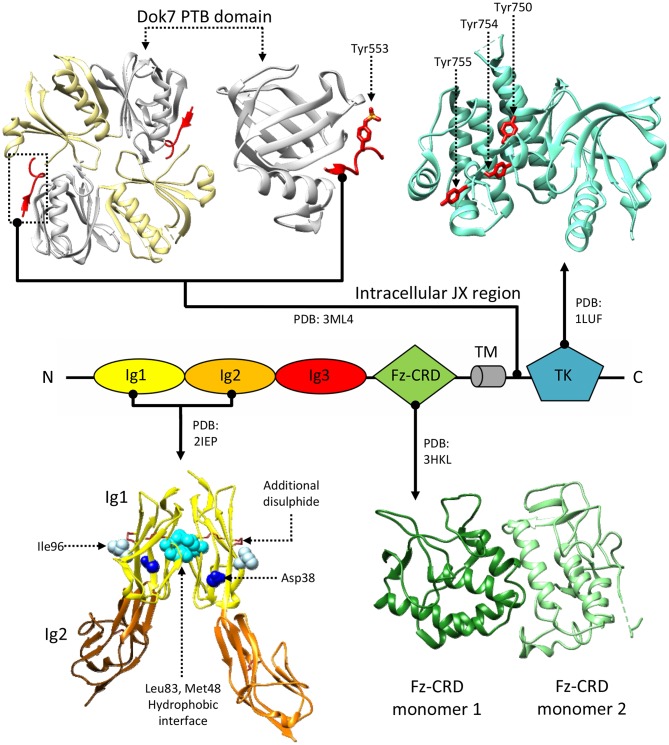
Figure 4.
Structural information about MuSK. MuSK interacts with the Dok7 (top-left) phosphotyrosine-binding domain (PTB, white) via a phosphorylated tyrosine (Tyr553) on its intracellular juxtamembrane (JX) region (red). The tyrosine kinase (TK) domain (top right) mediates intracellular signaling after phosphorylation of Tyr750, Tyr754, and Tyr755 (shown in red), located on a regulatory loop. Extracellular interactions with LRP4 are mediated by MuSK Ig domains. A crystal structure of an Ig1-Ig2 MuSK dimer (bottom left) shows a hydrophobic dimerization interface (cyan) reliant upon aminoacids Met48 and Leu83. Residues Asp38 (blue) and Ile96 (white), on the exposed surfaces of the Ig1 domains, are respectively involved in congenital myasthenia and LRP4 binding. Disulphide bonds stabilize both the Ig1 and Ig2 domain structures, the former has an additional disulphide bridge. Unlike the Ig domains, the function of the Fz-CRD domain (bottom-right) is extensively debated. The crystal structure presents two alternative conformations packed in an asymmetric dimer. Residue numbering refers to the reference structures.