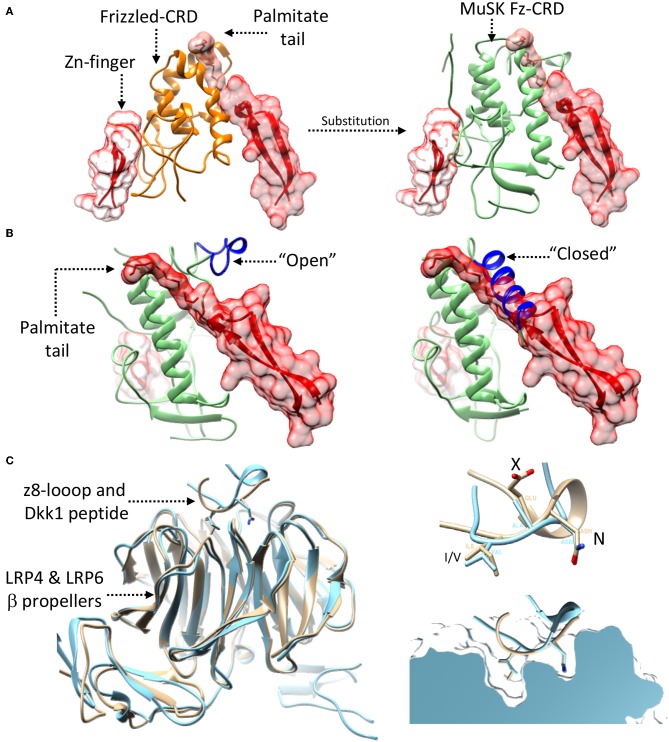
Figure 7.
Possibility of Wnt-agrin:LRP4:MuSK cross-talk. (A) The interaction (left-panel) between Wnt's (red) and Frizzled domains (orange) relies on two opposed interfaces that accommodate contemporaneously a palmitate tail and a Zn-finger domain. Substituting (right-panel) the Frizzled domain with the MuSK Fz-CRD (light-green) shows how this domain of MuSK could accommodate the same type of opposed binding interfaces. (B) The crystal structure of the MuSK Fz-CRD (also shown in Figure 4) presents two alternative conformations; an “open” conformation (left), possibly capable of accommodating the Wnt palmitate tail, and a “closed” one, likely not conductive to Wnt binding (right). “open” and “closed” states are determined the by the stabilization of an α-helix from an otherwise disordered loop (shown in blue). (C) A superposition (left) of the agrin:LRP4 z8 loop complex (cyan) with an LRP6:Dkk1 N-terminal peptide complex (beige) shows the structural similarity of the interaction, hinting the possibility of cross-talk. Both the agrin z8-loop and the Dkk1 peptide interact with their target via a conserved NxI/V motif (top-right). A cross section view into the binding pocket of LRP4 (top) for the agrin-z8 loop (cyan) shows how it could also accommodate the Dkk1 peptide (beige) and allow for cross-talk between the two signaling pathways.