Abstract
Patient-ventilator asynchrony (PVA) is common in patients receiving noninvasive ventilation (NIV). This occurs primarily when the triggering and cycling-off of ventilatory assistance are not synchronized with the patient's inspiratory efforts and could result in increased work of breathing and niv failure. In general, five types of asynchrony can occur during NIV: ineffective inspiratory efforts, double-triggering, auto-triggering, short-ventilatory cycling, and long-ventilatory cycling. Many factors that affect PVA are mostly related to the degree of air leakage, level of pressure support, and the type and properties of the interface used. Careful monitoring and adjustment of these factors are essential to reduce PVA and improve patient comfort. In this article, we discuss the machine and interface-related factors that influence PVA during NIV and its effect on the respiratory mechanics during pressure support ventilation, which is the ventilatory mode used most commonly during NIV. For that, we critically evaluated studies that assessed ventilator- and interface-related factors that influence PVA during NIV and proposed therapeutic solutions.
Keywords: Air leak, humidity, interface, noninvasive ventilation, patient-ventilator synchrony
Patient-ventilator asynchrony (PVA) describes a state of uncoordinated interactions between the patient and the ventilator. It occurs secondary to the mismatch between the patient's respiratory effort and the delivered ventilator support. This can lead to ineffective ventilation, increase work of breathing, and aggravate patient discomfort. Delays between the delivered ventilator breath and the patient's breathing effort can occur either at the beginning of the inspiratory cycle (triggering phase) or at the end of the inspiratory effort (cycling phase), resulting in PVA.[1]
PVA is frequently encountered during noninvasive ventilation (NIV). When measured with a global asynchrony index, PVA was observed in 24%–43% of patients with acute respiratory failure (ARF).[2] In a prospective multicenter observational study, Vignaux et al. observed severe asynchrony, (asynchrony index >10%) in 43% of patients receiving NIV for ARF.[3] The most important predictive factors for severe asynchrony were the level of pressure support and the magnitude of leaks.[3] The asynchrony index was calculated as the number of asynchrony events divided by the total respiratory rate, computed as the sum of the number of ventilator cycles (triggered or not) and wasted efforts: Asynchrony index (%) = number of asynchrony events/total respiratory rate × 100%.[3] Asynchrony events were determined by clinical observation as well as by recordings from surface diaphragmatic electromyography.[3]
During NIV, triggering and cycling-off of ventilatory assistance should ideally be synchronized with the patient's inspiratory efforts.[4] However, during positive airway pressure ventilation, there could be an inspiratory delay between the beginning of inspiratory effort and the start of positive inspiratory pressure.[4,5,6] Long inspiratory delays significantly increase the patient's work of breathing, since they delay the delivery of positive inspiratory pressure, and therefore, reduce the amount of assistance delivered to the patient during the early stages of the breathing effort.[7]
In general, five types of asynchrony can occur during NIV: ineffective inspiratory efforts, double-triggering, auto-triggering, short-ventilatory cycling, and long-ventilatory cycling. Ineffective inspiratory breathing occurs when the patient's inspiratory effort does not result in delivery of any breath. In double-triggering, two consecutive breaths occur with an interval of less than half of the mean inspiratory time. In auto-triggering, the machine delivers breaths in the absence of patient's effort, as inferred by the absence of a decrease in airway pressure prior to the machine-delivered breath.[8] Cycling asynchrony, whether short (premature) cycling or long (late) cycling, occurs when there is a mismatch between the inspiratory time that the patient demands, which is termed “neural inspiratory time,” and the inspiratory time provided by the ventilator. In premature cycling, the ventilator inappropriately cycles into expiration early, while the patient still in inspiration; the inspiratory time is <50% of the mean inspiratory time. On the other hand, “in delayed expiratory cycling,” the patient is ready to exhale, but the ventilator inappropriately continues to deliver an inspiratory breath and the inspiratory time exceeds twice the mean inspiratory time.[9,10]
In this article, we discuss the machine- and interface-related factors that influence PVA during NIV and its effect on the respiratory mechanics during pressure support ventilation, which is the ventilatory mode used most commonly during NIV. Pressure support ventilation is a patient-triggered and flow-cycled mode of ventilation. It allows patients to control the start of each breath. The maximum inspiratory pressure is set by the operator to deliver fixed pressure to the airways during inspiration. Airflow starts high to approach the targeted pressure rapidly, then gradually decreases as the airway pressure inside the alveoli builds up. Once a predefined percentage of the maximum inspiratory flow is reached (usually 25%), the expiratory valve opens and the inspiratory cycle is terminated. Several parameters can be adjusted with pressure support ventilation including the trigger-on threshold, the inspiratory rise time, the pressure level, and in some ventilators, the cycling-off airflow threshold. Figure 1 illustrates the pressure and flow waves of pressure support. Figure 2 illustrates pressure and flow waves of some of the common types of PVA during pressure support during.
Figure 1.
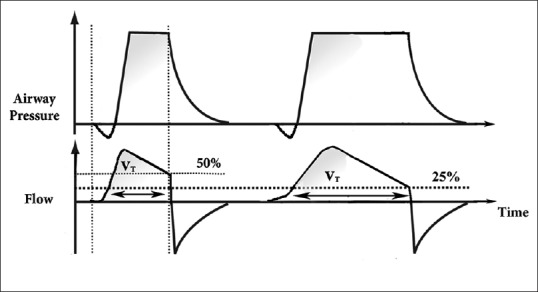
This illustration shows the pressure and flow waves of pressure support. The delivered tidal volume is calculated by the area under the flow curve wave. The inspiratory airflow continuously decreases because, as air moves inside the lungs, the pressure in the alveoli builds up resulting in a reduced pressure difference between the machine and the alveoli (ΔP), and hence a decelerating flow wave. Cycling-off starts when airflow reaches a preset threshold, which is a preset percentage of maximal air flow (usually 25%). At this point, the ventilator stops to deliver inspiratory flow, the expiratory valve opens to allow passive exhalation, and pressure goes down to the set positive end-expiratory pressure. Increasing the cycling-off preset percentage of maximal airflow results in decreasing the inspiratory cycle. The illustration demonstrates two cycles; one of them has cycling-off preset percentage of maximal airflow of 25%, and the other is 50%. The inspiratory time is shorter with 50%, and hence the delivered tidal volume
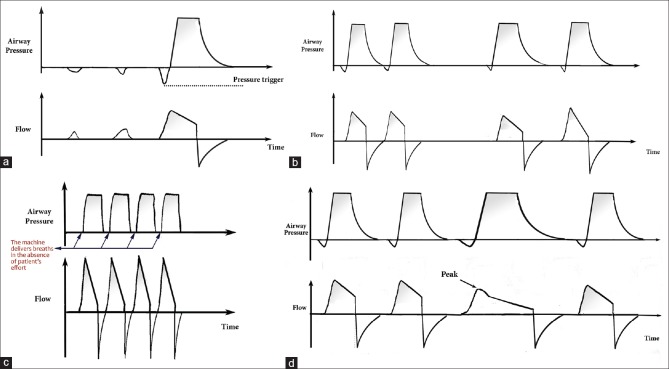
Figure 2.
It shows illustrations of pressure and flow waves of some of the common types of patient-ventilator synchrony during pressure support ventilation. (a) The figure illustrates pressure and flow waves during pressure support, where the patient tries to breathe, but he was unable to generate the needed effort required to trigger the ventilator. These minor efforts increase the work of breathing without generating enough tidal volume. (b) The figure illustrates pressure and flow waves during pressure support. When the ventilator does not meet the patients' demand for tidal volume, double-triggering may appear. In this example, two consecutive breaths occur with an interval of less than half of the mean inspiratory time. (c) The figure illustrates pressure and flow waves during pressure support. The machine delivers breaths in the absence of patient's effort, as inferred by the absence of a decrease in airway pressure prior to the machine-delivered breath. The illustration demonstrates the occurrence of more than three consecutive pressurizations at a ventilator frequency. (d) The figure illustrates pressure and flow waves during pressure support. The illustration shows an anomalous flow wave and an increased inspiratory time due to inspiratory air leak
Search Methods
A literature search was performed on April 1, 2018 with keyword searches for “NIV,” “air leak,” “asynchrony,” and “dyssynchrony” using PubMed (20 results), Google Scholar (47 results), and Medline (61 results). The reference lists of the identified articles were also searched for any additional sources. Publications were then filtered on the basis of whether or not they discussed the factors associated with PVA during NIV.
Patient-Related Factors That Influence Patient-Ventilator Asynchrony
The patient's physiological condition can influence patient-ventilator interaction. Patients with a low respiratory drive, weak inspiratory muscles, or dynamic hyperinflation causing intrinsic positive end-expiratory pressure can have ineffective breathing. Patients with restrictive lung disease have a short respiratory cycle and increased inspiratory time, resulting in premature cycling. On the other hand, patients with obstructive lung disease have a short inspiratory time and delayed cycling.[3]
Machine- and Interface-Related Factors
Table 1 shows the common factors affecting PVA. Table 2 presents the proposed solutions for some of the causes of PVA.
Table 1.
Factors affecting patient-ventilator synchrony
| Factor | Effects |
|---|---|
| Physiological condition of the patient | Restrictive lung disease: Due to rapid shallow breathing, it may lead to premature cycling Obstructive lung disease: Due to prolonged expiration (expiratory cycle), the inspiratory cycle shortens, resulting in a mismatch between the inspiratory time that the patient demands and this may lead to a delay in cycling |
| Air leak | Expiratory leak: Leads to auto-triggering Inspiratory leak: Leads to delays in cycling and reduced inspiratory sensitivity. During pressure support, significant leaks result in increases in the inspiratory flow in order to increase airway pressure to attain the set airway pressure. The large increase in inspiratory flow during leak compensation may result in significant side effects, including impaired mask seal and hence causing more leak, or gastric distension Leak leads to loss of PEEP and pressure support, and rebreathing |
| Level of pressure support | High-pressure support delays expiratory cycle and the ventilator breath will continue into the neural expiration Low-pressure support leads to early expiration and delayed triggering while the respiratory muscles are still contracting. This, in turn, leads to delayed triggering and wasted effort |
| Interface type | Can affect air leaks and mechanical dead space and CO2 accumulation and PVA Helmet can cause longer trigger delay and a shorter time of synchrony between mechanical support and patient inspiration compared with nasal and oronasal masks |
| Humidity | Low humidity can increase NAWR and mouth leak, which can lead to unsuccessful acclimatization to NIV in the chronic setting, and to the failure of NIV to improve gas exchange and dyspnea in the acute setting |
PVA=Patient-ventilator synchrony, NIV=Noninvasive ventilation, PEEP=Positive end-expiratory pressure, NAWR=Nasal airway resistance
Table 2.
Proposed solutions for some of the causes of patient-ventilator asynchrony
| Cause | Description | Solution |
|---|---|---|
| Auto-triggering | It is defined as the occurrence of at least three consecutive pressurizations at a ventilator frequency[8] of >40/min not synchronized with patient respiration The trigger is too sensitive or nonrespiratory factors trigger the ventilator such as Cardiac contractions: This may cause a small amount of air movement, and if the flow trigger is sensitive enough, this air movement can trigger the ventilator breaths. The respiratory rate may match the heart rate Leak from the circuit or from the chest drain (e.g., a bronchopleural fistula) Inappropriate sensitivity settings Excessive water condensation in the ventilator circuit Large volume of respiratory secretions Swallowing or vomiting Peristalsis in a massive hiatus hernia or intrathoracic bowel loops Muscle contractions due to external pacing |
Remove the cause such as excessive water condensation or respiratory secretions If no treatable cause is detected, adjust the trigger to a higher setting |
| Double-triggering | When an insufficient level of pressure support is applied or the patient’s demand is high, the inspiratory effort may continue throughout the preset ventilator inspiratory time and result in retriggering of the ventilator after it has discontinued pressurization, which may lead to the delivery of two cycles for only one patient’s effort (double-triggering) | Adjust the expiratory flow trigger until the desired tidal volume is achieved |
| Large leak around the NIV mask | In order to generate the specified pressure, the ventilator continues to deliver high flow. With a large leak, inspiration can be very uncomfortable (as the ventilator delivers 70-80 L/min of gas into the patients face)[5] | Adjust the mask to minimize the leak Decreasing the level of pressure support will decrease the total inspiratory time, as the machine will cycle to expiration sooner In some ventilators, one can actually adjust the inspiratory time directly |
| Wasted effort | Wasted efforts can occur during inspiration when the patient tries to initiate a breath (straining to inhale against a closed inspiratory valve) Possible causes Respiratory muscle weakness Reduced respiratory drive Inadequate trigger threshold setting iPEEP: Inspiratory muscles have to overcome iPEEP and trigger sensitivity to trigger the ventilator Inadequate level of support: The flow rate is too low and it does not meet patient demand |
The use of ePEEP to approximately 80%-90% of the iPEEP can counterbalance iPEEP and as such facilitate triggering Reduce the trigger sensitivity |
PEEP=Positive end-expiratory pressure, ePEEP=Extrinsic PEEP, iPEEP=Intrinsic-PEEP, NIV=Noninvasive ventilation
The effect of air leaks on patient-ventilator asynchrony
To meet the patient's demand and reduce the work of breathing and discomfort, the ventilator must have an efficient triggering and pressurization capacity. The sensitivity of the inspiratory trigger is a key element that depends on the sensing technology and the sealing level of the inspiratory circuit. The presence of leaks may principally affect the trigger and cycle-off phases.[11] Most ventilators use algorithms to compensate for air leak. During pressure support, significant leaks result in increases in the inspiratory flow in order to increase airway pressure to attain the set airway pressure.[7,12] The large increase in inspiratory flow during leak compensation may result in significant side effects, including impairing mask seal and hence causing more leak or causing gastric distension.[13]
Significant leaks may result in a pressure drop or produce flows that are interpreted by the ventilator as a patient inspiratory effort, thus causing auto-triggering. This can only be resolved by reducing triggering sensitivity, which in turn creates late cycling or failed triggering, as reported in 12%–23% of patients on NIV.[7] The presence of an inspiratory leak works as sustained inspiration and therefore will delay the cycle.[11,14]
The manner in which leaks can affect patient-ventilator interaction is influenced not only by the amount of leakage, but also by the ventilator's leak compensation features.[3] In a bench study, Carteaux et al. reported that dedicated NIV ventilators used in critically ill patients were associated with a lower incidence of PVA than intensive care unit (ICU) and transport ventilators, even when the NIV algorithm was used to minimize the asynchrony.[15] Large air leaks can lead to loss of extrinsic positive end-expiratory pressure (ePEEP) and pressure support, and an increase in ventilator auto-triggering, rebreathing of exhaled gas, and therefore, increased PVA. This may result in a decrease in FiO2 and oxygen saturation and NIV failure.[13,16] Ueno et al., studied the difference between the set and actual pressure support (ΔPS), and between the set and actual PEEP level (ΔPEEP) during NIV at different leak levels.[17] The investigators found that the differences (ΔPS and Δ PEEP) were less in dedicated NIV ventilators than ICU ventilators at medium leak levels.[17] However, with large air leaks, the set pressure support could not be adequately maintained in all ventilators. Readjustment of triggering sensitivity and cycling criteria was necessary to avoid auto-triggering with the ICU ventilators, but not with dedicated NIV ventilators.[17,18] In addition, with NIV ventilators, the trigger pressure time product (PTP), which is the area under the pressure-time curve between the onset of inspiratory effort and the return to PEEP, increased significantly at higher leak levels.[5,17,19,20] PEEP settings and airway resistance levels did not affect trigger PTP. The trigger PTP reflects both the sensitivity of the ventilator in detecting inspiratory effort and the ventilator's ability to deliver high flow at the onset of inspiration.[13,17] Inspiratory PTP, on the other hand, decreased in all ventilators with each successive leak level.[13] This reduction in inspiratory PTP is related to impaired pressurization rate, and the reduced ventilator capacity to maintain the set pressure during inspiration.[5,17,19,20] This, in turn, can result in an increase in patient inspiratory effort.[5,17,19,20]
In general, ICU ventilators and NIV dedicated ventilators show important differences with regard to leak compensation.[21] In a bench study of eight ICU ventilators featuring a NIV mode, Vignaux et al. found that in most of the tested ventilators, leaks led to an increase in the trigger delay and a decrease in the ability to reach the pressure target and delayed cycling.[3] On the other hand, NIV dedicated ventilators allowed better synchronization than ICU ventilators in the presence of leaks[14,22] and were triggered properly at all levels of air leakage. The inspiratory triggering of NIV dedicated ventilators was more effective than that with the ICU ventilators.[22,23] This favors its use over invasive ICU ventilators.[16] In addition, different masks have various leak levels. Therefore, trigger sensitivity, pressure level, and rebreathing must be checked when switching to a mask that has a different leak.[11,24,25] Nevertheless, the above findings are not universal. Oto et al. tested the ability of seven ICU ventilators and one NIV dedicated ventilator to prevent triggering and cycling asynchrony secondary to different leak levels.[21] In contrast to the previous studies, their study showed that one of the ICU ventilators outperformed the NIV dedicated ventilator and was the fastest to stabilize and synchronize ventilation with different levels of leakage.[21] This highlights the need for further improvement in the technical aspects of ventilators to help the treating team manage ventilator leaks.
Intentional leaks (mask or circuit leaks to remove CO2 from the interface), even when applied according to manufacturer's instructions, could affect patient-ventilator synchrony. In a bench study, Louis et al. examined the performance of four ventilators with their recommended masks and with masks showing the largest and smallest leak levels while maintaining the same setting.[26] The results showed that the mask with the largest leak was associated with auto-triggering and/or decreased inspiratory trigger sensitivity with the majority of ventilators. On the other hand, the mask with the smallest intentional leak was associated with increased rebreathing. Hence, trigger sensitivity, pressurization level, and rebreathing must be checked when switching to a mask that has a different leak.
Pressure support and patient-ventilator asynchrony
In addition to air leaks, which are a major factor affecting PVA, the pressure support level and delivered tidal volume are also important.[27] For example, high-pressure support delays the expiratory cycle and the ventilator breath will continue into the neural expiration. On the other hand, low-pressure support can lead to early expiration while the respiratory muscles are still contracting. This leads to delayed triggering and wasted effort.[3,11,28]
Recently, neurally adjusted ventilator assist (NAVA), which uses the diaphragm electrical activity to trigger and drive ventilator assistance, have been shown to improve patient-ventilator interaction and synchrony during NIV, with no difference in gas exchange, respiratory rate, and neural drive and timing.[4,29] This is probably due to the fewer trigger and cycling delays, while the patient is allowed to breathe spontaneously.[4] A specific setting to generate neurally controlled pressure support (PSVN) was recently proposed for delivering NIV by helmet.[30]
When PSVN was compared with the standard pneumatically triggered and cycled-off pressure support (PSVP) and NAVA during NIV using a facial mask in 14 patients postextubation, PSVN resulted in significantly improved comfort and patient-ventilator interaction during NIV by a facial mask compared to PSVP and NAVA. PSVN also improved synchrony, as opposed to PSVP only.[31] Compared to PSVP and NAVA, PSVN reduced the peak inspiratory flow time (P < 0.001).[31]
Olivieri et al. reviewed bench studies that assessed the performance of ventilators and interfaces during NIV.[32] They highlighted some specific technical aspects that may limit the application of the results of bench studies to clinical practice.[11,32] For example, there is a lack of standard references for simulated demand and effort, mode of generation and extent of air leaks, resistance and compliance of the virtual respiratory system, and ventilator settings in the different lung models used in the studies. The extent of air leaks varied widely among studies, ranging between 6 L/min and 120 L/min.[11,32] Therefore, more practice-related clinical studies are needed.
Effects of interfaces on patient-ventilator synchrony
Interfaces used for ventilation could affect patient-ventilator interaction as they influence the degree of air leakage, which in turn reduces the efficiency of NIV. In addition, different interfaces can increase mechanical dead space, which is around 205 ml with an oronasal mask and 120 ml with a nasal mask in vitro.[11] This can lead to CO2 accumulation and PVA. Nasal or oronasal masks seem to cause less PVA than a mouthpiece or helmet. Costa et al. evaluated patient-ventilator interactions during pressure support ventilation delivered via invasive ventilation (endotracheal tube) and NIV via an oronasal mask or helmet.[33] This bench study demonstrated that synchrony was significantly better with an endotracheal tube. Conversely, when compared with an oronasal mask, the helmet showed worse synchrony due to a longer trigger delay and a shorter time of synchrony between mechanical support and patient inspiration.[33] Studies performed in healthy volunteers and in patients with stable chronic obstructive pulmonary disease showed that helmet ventilation was less efficient in decreasing inspiratory effort compared to conventional face masks, increasing the likelihood of PVA, especially related to the triggering delay.[6,34] Of all available interfaces, the helmet probably shows more problems with synchrony between patient breathing and ventilator cycling because of its soft compliant wall and high inner volume. In a lung model study, Moerer et al. showed that the delay times were more than twice as long with the helmet compared with NIV via face mask or during invasive ventilation, but the delay decreased with increasing continuous positive airway pressure (CPAP) or pressure support levels.[35] In addition, Vargas et al. demonstrated that both ePEEP and pressure support should be increased by 50% when the helmet is used to achieve the same effects of NIV delivered by the face mask.[36] The specific settings significantly improved inspiratory-muscle unloading.[36] The latter results suggest that the increased work of breathing observed with helmet ventilation is explained in part by trigger and pressurization difficulties, and not solely by a large dead space problem. Accordingly, Costa et al. found that during noninvasive pressure support ventilation delivered by helmet, a ventilator setting characterized by a fast inspiratory ramp and expiratory trigger could significantly reduce PVA.[33] Similarly, Racca et al. demonstrated an increase in inspiratory effort and dyspnea and lower CO2 clearance when a helmet was used for the delivery of pressure support ventilation compared to a face mask.[6,37]
Recently, a new novel helmet (NH) characterized by an annular openable ring placed underneath an inflatable cushion that secures the helmet without the need for armpit braces has been introduced into clinical practice. The NH has demonstrated improved comfort and patient-ventilator interaction in healthy volunteers and in bench studies.[32,38] Olivieri et al. compared the NH with the standard helmet in 14 ICU patients who used NIV for prevention of postextubation failure. The NH significantly improved comfort and inspiratory trigger delay and pressurization.[39]
Effect of humidity on patient-ventilator synchrony
NIV is usually delivered through a nasal or oronasal mask; when the inspired gas passes through the upper airway, it gets conditioned. Therefore, patients under NIV require adequate humidification and heating of inspired air (this is called gas conditioning).[40,41] Air leaks through the mouth or peripheral masks can result in a unidirectional nasal airflow; therefore, the mucosa recovers less heat and moisture during expiration. This may cause a continuous drop in airway humidity.[42]
Clinically, increased nasal airway resistance (NAWR) is likely to lead to unsuccessful acclimatization to NIV in the chronic setting and to the failure of NIV to improve gas exchange and dyspnea in the acute setting. An increase in NAWR due to a large mouth air leak when using a nasal mask during NIV can occur in response to prolonged inhalation of dry air.[41,42]
In general, nasal masks tend to show more air leaks than face masks. Nasal masks promote mouth leaks, which result in high unidirectional nasal flow and increased nasal resistance and mouth opening. This, in turn, perpetuates mouth leaks.[43,44,45,46] In a study in adult volunteers, a nasal CPAP with a mouth leak resulted in a three-fold increase in NAWR that was significantly reduced by adequate humidification.[44,47] Heated humidification has been shown to reduce nasal resistance by increasing the relative humidity (RH) of the inhaled air.[41]
Besides the increase in NAWR, inefficient gas conditioning during NIV can cause structural and functional damage to the nasal mucosa including, the ciliary activity, mucus secretion, and local blood flow. Nasal congestion and nasal/oral dryness affect 10%–20% of patients during NIV, particularly when a nasal mask is used.[5,11,28] Nasal or oral dryness is usually indicative of air leaks through the mouth with consequent loss of the nasal mucosa's capacity to heat and to humidify the inspired air. If the air leak persists, the nasal mucosa progressively dries and releases inflammatory mediators that increase nasal congestion and resistance. This can cause reduced tidal volume (VT), increased work of breathing, patient discomfort, asynchrony, and poor compliance with NIV. These findings were confirmed in adult volunteers undergoing NIV using nasal CPAP.[43,44]
Heated humidification reduces nasal resistance by increasing the RH of the inhaled air. Humidification can also be provided by passive heat-and-moisture exchanger (HME) methods. Several studies, however, have shown an increase in minute ventilation and work of breathing with HME-based methods compared to heated humidifier.[48,49,50] In addition, Jaber et al. reported higher PaCO2 with an HME than with a heated humidifier, which is probably related to the greater dead space with an HME.[51]
The resultant increase in workload and possible CO2 accumulation can adversely affect patient-ventilator interaction and discourage the use of an HME during NIV.[45,52,53]
Conclusions
This work tried to gather all important studies that addressed PVA during NIV and present data in a simple, legible way using illustrations when needed. Nevertheless, careful monitoring and check are necessary to apply the gained knowledge and develop the needed skills to diagnose and treat PVA during NIV.
Air leaks are the most important factor affecting PVA; expiratory leaks lead to auto-triggering while inspiratory leaks can delay cycling and reduce inspiratory sensitivity. The level of pressure support and the amount of delivered tidal volume are additional factors that can interfere with the expiratory cycle. The use of NAVA is promising, as it may reduce triggering and cycling delay. The type and size of an interface can affect air leak and mechanical dead space. To reduce PVA, nasal or oronasal masks are preferred over mouthpieces or helmets. The addition of humidification can further reduce NAWR and increase patient compliance with NIV.
PVA remains a major obstacle to improve NIV utility. Future research is needed to develop software-based NIV setting or a new “fully noninvasive” triggering system that can improve patient-ventilator synchrony, correct auto-triggering, and avoid wasted efforts in the presence of air leaks.[29,54]
Financial support and sponsorship
This project was partially funded by The Strategic Technologies Program of the National Plan for Sciences and Technology and Innovation in the Kingdom of Saudi Arabia (08-MED511-02).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Tobin MJ, Jubran A, Laghi F. Patient-ventilator interaction. Am J Respir Crit Care Med. 2001;163:1059–63. doi: 10.1164/ajrccm.163.5.2005125. [DOI] [PubMed] [Google Scholar]
- 2.Longhini F, Colombo D, Pisani L, Idone F, Chun P, Doorduin J, et al. Efficacy of ventilator waveform observation for detection of patient-ventilator asynchrony during NIV: A multicentre study. ERJ Open Res. 2017;3:pii: 00075–2017. doi: 10.1183/23120541.00075-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vignaux L, Vargas F, Roeseler J, Tassaux D, Thille AW, Kossowsky MP, et al. Patient-ventilator asynchrony during non-invasive ventilation for acute respiratory failure: A multicenter study. Intensive Care Med. 2009;35:840–6. doi: 10.1007/s00134-009-1416-5. [DOI] [PubMed] [Google Scholar]
- 4.Spahija J, de Marchie M, Albert M, Bellemare P, Delisle S, Beck J, et al. Patient-ventilator interaction during pressure support ventilation and neurally adjusted ventilatory assist. Crit Care Med. 2010;38:518–26. doi: 10.1097/CCM.0b013e3181cb0d7b. [DOI] [PubMed] [Google Scholar]
- 5.Carron M, Freo U, BaHammam AS, Dellweg D, Guarracino F, Cosentini R, et al. Complications of non-invasive ventilation techniques: A comprehensive qualitative review of randomized trials. Br J Anaesth. 2013;110:896–914. doi: 10.1093/bja/aet070. [DOI] [PubMed] [Google Scholar]
- 6.Racca F, Appendini L, Gregoretti C, Stra E, Patessio A, Donner CF, et al. Effectiveness of mask and helmet interfaces to deliver noninvasive ventilation in a human model of resistive breathing. J Appl Physiol (1985) 2005;99:1262–71. doi: 10.1152/japplphysiol.01363.2004. [DOI] [PubMed] [Google Scholar]
- 7.De Luca A, Sall FS, Khoury A. Leak compensation algorithms: The key remedy to noninvasive ventilation failure? Respir Care. 2017;62:135–6. doi: 10.4187/respcare.05289. [DOI] [PubMed] [Google Scholar]
- 8.Guo YF, Sforza E, Janssens JP. Respiratory patterns during sleep in obesity-hypoventilation patients treated with nocturnal pressure support: A preliminary report. Chest. 2007;131:1090–9. doi: 10.1378/chest.06-1705. [DOI] [PubMed] [Google Scholar]
- 9.Ramsay M. Patient ventilator asynchrony and sleep disruption during non-invasive ventilation. J Thorac Dis. 2018;10:S80–5. doi: 10.21037/jtd.2017.11.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garofalo E, Bruni A, Pelaia C, Liparota L, Lombardo N, Longhini F, et al. Recognizing, quantifying and managing patient-ventilator asynchrony in invasive and noninvasive ventilation. Expert Rev Respir Med. 2018;12:557–67. doi: 10.1080/17476348.2018.1480941. [DOI] [PubMed] [Google Scholar]
- 11.Pisani L, Carlucci A, Nava S. Interfaces for noninvasive mechanical ventilation: Technical aspects and efficiency. Minerva Anestesiol. 2012;78:1154–61. [PubMed] [Google Scholar]
- 12.Mehta S, Hill NS. Noninvasive ventilation. Am J Respir Crit Care Med. 2001;163:540–77. doi: 10.1164/ajrccm.163.2.9906116. [DOI] [PubMed] [Google Scholar]
- 13.Storre JH, Bohm P, Dreher M, Windisch W. Clinical impact of leak compensation during non-invasive ventilation. Respir Med. 2009;103:1477–83. doi: 10.1016/j.rmed.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 14.Schettino GP, Tucci MR, Sousa R, Valente Barbas CS, Passos Amato MB, Carvalho CR. Mask mechanics and leak dynamics during noninvasive pressure support ventilation: A bench study. Intensive Care Med. 2001;27:1887–91. doi: 10.1007/s00134-001-1146-9. [DOI] [PubMed] [Google Scholar]
- 15.Carteaux G, Lyazidi A, Cordoba-Izquierdo A, Vignaux L, Jolliet P, Thille AW, et al. Patient-ventilator asynchrony during noninvasive ventilation: A bench and clinical study. Chest. 2012;142:367–76. doi: 10.1378/chest.11-2279. [DOI] [PubMed] [Google Scholar]
- 16.Miyoshi E, Fujino Y, Uchiyama A, Mashimo T, Nishimura M. Effects of gas leak on triggering function, humidification, and inspiratory oxygen fraction during noninvasive positive airway pressure ventilation. Chest. 2005;128:3691–8. doi: 10.1378/chest.128.5.3691. [DOI] [PubMed] [Google Scholar]
- 17.Ueno Y, Nakanishi N, Oto J, Imanaka H, Nishimura M. A bench study of the effects of leak on ventilator performance during noninvasive ventilation. Respir Care. 2011;56:1758–64. doi: 10.4187/respcare.01145. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira JC, Chipman DW, Hill NS, Kacmarek RM. Bilevel vs. ICU ventilators providing noninvasive ventilation: Effect of system leaks: A COPD lung model comparison. Chest. 2009;136:448–56. doi: 10.1378/chest.08-3018. [DOI] [PubMed] [Google Scholar]
- 19.Chatburn RL. Which ventilators and modes can be used to deliver noninvasive ventilation? Respir Care. 2009;54:85–101. [PubMed] [Google Scholar]
- 20.Murata S, Yokoyama K, Sakamoto Y, Yamashita K, Oto J, Imanaka H, et al. Effects of inspiratory rise time on triggering work load during pressure-support ventilation: A lung model study. Respir Care. 2010;55:878–84. [PubMed] [Google Scholar]
- 21.Oto J, Chenelle CT, Marchese AD, Kacmarek RM. A comparison of leak compensation in acute care ventilators during noninvasive and invasive ventilation: A lung model study. Respir Care. 2013;58:2027–37. doi: 10.4187/respcare.02466. [DOI] [PubMed] [Google Scholar]
- 22.Schönhofer B, Sortor-Leger S. Equipment needs for noninvasive mechanical ventilation. Eur Respir J. 2002;20:1029–36. doi: 10.1183/09031936.02.00404202. [DOI] [PubMed] [Google Scholar]
- 23.Schettino GP, Chatmongkolchart S, Hess DR, Kacmarek RM. Position of exhalation port and mask design affect CO2 rebreathing during noninvasive positive pressure ventilation. Crit Care Med. 2003;31:2178–82. doi: 10.1097/01.CCM.0000081309.71887.E9. [DOI] [PubMed] [Google Scholar]
- 24.Elliott MW. The interface: Crucial for successful noninvasive ventilation. Eur Respir J. 2004;23:7–8. doi: 10.1183/09031936.03.00115903. [DOI] [PubMed] [Google Scholar]
- 25.Willson GN, Piper AJ, Norman M, Chaseling WG, Milross MA, Collins ER, et al. Nasal versus full face mask for noninvasive ventilation in chronic respiratory failure. Eur Respir J. 2004;23:605–9. doi: 10.1183/09031936.04.00051604. [DOI] [PubMed] [Google Scholar]
- 26.Louis B, Leroux K, Isabey D, Fauroux B, Lofaso F. Effect of manufacturer-inserted mask leaks on ventilator performance. Eur Respir J. 2010;35:627–36. doi: 10.1183/09031936.00188708. [DOI] [PubMed] [Google Scholar]
- 27.Thille AW, Cabello B, Galia F, Lyazidi A, Brochard L. Reduction of patient-ventilator asynchrony by reducing tidal volume during pressure-support ventilation. Intensive Care Med. 2008;34:1477–86. doi: 10.1007/s00134-008-1121-9. [DOI] [PubMed] [Google Scholar]
- 28.BaHammam AS, Singh TD, Gupta R, Pandi-Perumal SR. Choosing the proper interface for positive airway pressure therapy in subjects with acute respiratory failure. Respir Care. 2018;63:227–37. doi: 10.4187/respcare.05787. [DOI] [PubMed] [Google Scholar]
- 29.Cammarota G, Olivieri C, Costa R, Vaschetto R, Colombo D, Turucz E, et al. Noninvasive ventilation through a helmet in postextubation hypoxemic patients: Physiologic comparison between neurally adjusted ventilatory assist and pressure support ventilation. Intensive Care Med. 2011;37:1943–50. doi: 10.1007/s00134-011-2382-2. [DOI] [PubMed] [Google Scholar]
- 30.Cammarota G, Longhini F, Perucca R, Ronco C, Colombo D, Messina A, et al. New setting of neurally adjusted ventilatory assist during noninvasive ventilation through a helmet. Anesthesiology. 2016;125:1181–9. doi: 10.1097/ALN.0000000000001354. [DOI] [PubMed] [Google Scholar]
- 31.Longhini F, Pan C, Xie J, Cammarota G, Bruni A, Garofalo E, et al. New setting of neurally adjusted ventilatory assist for noninvasive ventilation by facial mask: A physiologic study. Crit Care. 2017;21:170. doi: 10.1186/s13054-017-1761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olivieri C, Costa R, Conti G, Navalesi P. Bench studies evaluating devices for non-invasive ventilation: Critical analysis and future perspectives. Intensive Care Med. 2012;38:160–7. doi: 10.1007/s00134-011-2416-9. [DOI] [PubMed] [Google Scholar]
- 33.Costa R, Navalesi P, Spinazzola G, Ferrone G, Pellegrini A, Cavaliere F, et al. Influence of ventilator settings on patient-ventilator synchrony during pressure support ventilation with different interfaces. Intensive Care Med. 2010;36:1363–70. doi: 10.1007/s00134-010-1915-4. [DOI] [PubMed] [Google Scholar]
- 34.Navalesi P, Costa R, Ceriana P, Carlucci A, Prinianakis G, Antonelli M, et al. Non-invasive ventilation in chronic obstructive pulmonary disease patients: Helmet versus facial mask. Intensive Care Med. 2007;33:74–81. doi: 10.1007/s00134-006-0391-3. [DOI] [PubMed] [Google Scholar]
- 35.Moerer O, Fischer S, Hartelt M, Kuvaki B, Quintel M, Neumann P. Influence of two different interfaces for noninvasive ventilation compared to invasive ventilation on the mechanical properties and performance of a respiratory system: A lung model study. Chest. 2006;129:1424–31. doi: 10.1378/chest.129.6.1424. [DOI] [PubMed] [Google Scholar]
- 36.Vargas F, Thille A, Lyazidi A, Campo FR, Brochard L. Helmet with specific settings versus facemask for noninvasive ventilation. Crit Care Med. 2009;37:1921–8. doi: 10.1097/CCM.0b013e31819fff93. [DOI] [PubMed] [Google Scholar]
- 37.Hess DR. Patient-ventilator interaction during noninvasive ventilation. Respir Care. 2011;56:153–65. doi: 10.4187/respcare.01049. [DOI] [PubMed] [Google Scholar]
- 38.Ferrone G, Cipriani F, Spinazzola G, Festa O, Arcangeli A, Proietti R, et al. Abench study of 2 ventilator circuits during helmet noninvasive ventilation. Respir Care. 2013;58:1474–81. doi: 10.4187/respcare.02060. [DOI] [PubMed] [Google Scholar]
- 39.Olivieri C, Longhini F, Cena T, Cammarota G, Vaschetto R, Messina A, et al. New versus conventional helmet for delivering noninvasive ventilation: A physiologic, crossover randomized study in critically ill patients. Anesthesiology. 2016;124:101–8. doi: 10.1097/ALN.0000000000000910. [DOI] [PubMed] [Google Scholar]
- 40.Evans TW. International consensus conferences in intensive care medicine: Non-invasive positive pressure ventilation in acute respiratory failure. Organised jointly by the American Thoracic Society, the European Respiratory Society, the European Society of Intensive Care Medicine, and the Société de Réanimation de Langue Française, and approved by the ATS board of directors, december 2000. Intensive Care Med. 2001;27:166–78. doi: 10.1007/s001340000721. [DOI] [PubMed] [Google Scholar]
- 41.Esquinas Rodriguez AM, Scala R, Soroksky A, BaHammam A, de Klerk A, Valipour A, et al. Clinical review: Humidifiers during non-invasive ventilation – Key topics and practical implications. Crit Care. 2012;16:203. doi: 10.1186/cc10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayes MJ, McGregor FB, Roberts DN, Schroter RC, Pride NB. Continuous nasal positive airway pressure with a mouth leak: Effect on nasal mucosal blood flux and nasal geometry. Thorax. 1995;50:1179–82. doi: 10.1136/thx.50.11.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carrey Z, Gottfried SB, Levy RD. Ventilatory muscle support in respiratory failure with nasal positive pressure ventilation. Chest. 1990;97:150–8. doi: 10.1378/chest.97.1.150. [DOI] [PubMed] [Google Scholar]
- 44.Richards GN, Cistulli PA, Ungar RG, Berthon-Jones M, Sullivan CE. Mouth leak with nasal continuous positive airway pressure increases nasal airway resistance. Am J Respir Crit Care Med. 1996;154:182–6. doi: 10.1164/ajrccm.154.1.8680678. [DOI] [PubMed] [Google Scholar]
- 45.Branson RD, Gentile MA. Is humidification always necessary during noninvasive ventilation in the hospital? Respir Care. 2010;55:209–16. [PubMed] [Google Scholar]
- 46.Nava S, Navalesi P, Gregoretti C. Interfaces and humidification for noninvasive mechanical ventilation. Respir Care. 2009;54:71–84. [PubMed] [Google Scholar]
- 47.Fischer Y, Keck T, Leiacker R, Rozsasi A, Rettinger G, Gruen PM. Effects of nasal mask leak and heated humidification on nasal mucosa in the therapy with nasal continuous positive airway pressure (nCPAP) Sleep Breath. 2008;12:353–7. doi: 10.1007/s11325-008-0173-y. [DOI] [PubMed] [Google Scholar]
- 48.Ari A, Dang T, Al Enazi FH, Alqahtani MM, Alkhathami A, Qoutah R, et al. Effect of heat moisture exchanger on aerosol drug delivery and airway resistance in simulated ventilator-dependent adults using jet and mesh nebulizers. J Aerosol Med Pulm Drug Deliv. 2018;31:42–8. doi: 10.1089/jamp.2016.1347. [DOI] [PubMed] [Google Scholar]
- 49.Wilkes AR. Heat and moisture exchangers and breathing system filters: Their use in anaesthesia and intensive care. Part 2 – Practical use, including problems, and their use with paediatric patients. Anaesthesia. 2011;66:40–51. doi: 10.1111/j.1365-2044.2010.06564.x. [DOI] [PubMed] [Google Scholar]
- 50.Lucato JJ, Cunha TM, Reis AM, Picanço PS, Barbosa RC, Liberali J, et al. Ventilatory changes during the use of heat and moisture exchangers in patients submitted to mechanical ventilation with support pressure and adjustments in ventilation parameters to compensate for these possible changes: A self-controlled intervention study in humans. Rev Bras Ter Intensiva. 2017;29:163–70. doi: 10.5935/0103-507X.20170026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jaber S, Chanques G, Matecki S, Ramonatxo M, Souche B, Perrigault PF, et al. Comparison of the effects of heat and moisture exchangers and heated humidifiers on ventilation and gas exchange during non-invasive ventilation. Intensive Care Med. 2002;28:1590–4. doi: 10.1007/s00134-002-1441-0. [DOI] [PubMed] [Google Scholar]
- 52.Lellouche F, Maggiore SM, Deye N, Taillé S, Pigeot J, Harf A, et al. Effect of the humidification device on the work of breathing during noninvasive ventilation. Intensive Care Med. 2002;28:1582–9. doi: 10.1007/s00134-002-1518-9. [DOI] [PubMed] [Google Scholar]
- 53.Lellouche F, Brochard L. Advanced closed loops during mechanical ventilation (PAV, NAVA, ASV, smartCare) Best Pract Res Clin Anaesthesiol. 2009;23:81–93. doi: 10.1016/j.bpa.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 54.Cortegiani A, Russotto V, Antonelli M, Azoulay E, Carlucci A, Conti G, et al. Ten important articles on noninvasive ventilation in critically ill patients and insights for the future: A report of expert opinions. BMC Anesthesiol. 2017;17:122. doi: 10.1186/s12871-017-0409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]