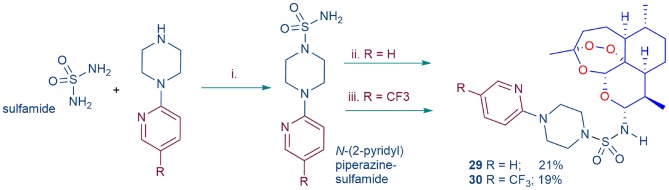
Scheme 2.
Preparation of substituted analogs of DHA-sulfamide 11 (Figure 2). i. Sulfamide (1.0 equiv.), N-(2-pyridyl) piperazine, or N-(4-trifluoromethyl-2-pyridyl) piperazine (1.0 equiv.), dimethoxyethane, reflux; ii. DHA-TMS ether, TMSBr in dichloromethane (Haynes et al., 2003, 2004), then N-(2-pyridyl)piperazine sulfamide (R = H); iii. DHA, COCl2-DMSO in toluene (Chan et al., 2018; Wu et al., 2018), then N-(4-trifluoromethyl-2-pyridyl)piperazine sulfamide (R = CF3); yields based on amount of DHA-TMS ether or DHA used.