Table 1.
Structures, eeAChE and eqBChE inhibitory activities of target compounds.
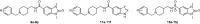 | ||||||
|---|---|---|---|---|---|---|
| Compound | R | X | Y | AChEa (IC50c, μM or IRd, %) | BChEb (IC50, μM or IR, %) | SIe |
| 9a | H | – | – | 3.14 ± 1.12 | 43.89 % | – |
| 9b | 2-CH3 | – | – | 8.08 ± 2.66 | 41.36 % | – |
| 9c | 3-CH3 | – | – | 3.83 ± 2.04 | 44.03 % | – |
| 9d | 4-CH3 | – | – | 25.71 ± 12.31 | 37.80 % | – |
| 9e | 3,4-CH3 | – | – | 9.97 ± 2.52 | 30.80 % | – |
| 9f | 3-OCH3 | – | – | 33.47 % | 25.02 % | – |
| 9g | 4-OCH3 | – | – | 5.17 ± 2.25 | 41.46 % | – |
| 9h | 2-F | – | – | 16.19 ± 4.34 | 30.01 % | – |
| 9i | 3-F | – | – | 2.93 ± 1.96 | 95.07 ± 59.26 | 0.24 |
| 9j | 4-F | – | – | 4.70 ± 2.01 | 3.71 ± 1.88 | 1.27 |
| 9k | 2-Cl | – | – | 3.92 ± 1.20 | 46.48 % | – |
| 9l | 3-Cl | – | – | 6.00 ± 3.31 | 36.93 % | – |
| 9m | 4-Cl | – | – | 0.21 ± 0.03 | 49.93 % | – |
| 9n | 2-Br | – | – | 8.87 ± 4.56 | 43.48 % | – |
| 9o | 3-Br | – | – | 3.69 ± 1.83 | 42.83 % | – |
| 9p | 4-Br | – | – | 11.06 ± 6.42 | 38.48 % | – |
| 11a | 4-OCH3 | CH | CH | 38.08 ± 12.83 | 10.75 ± 5.55 | 3.54 |
| 11b | 4-OCH3 | N | CH | 48.57 % | 44.57 % | – |
| 11c | 2-Cl | N | CH | 5.44 ± 2.22 | 21.29 ± 5.00 | 0.26 |
| 11d | 4-OCH3 | CH | N | 18.81 % | 16.51 % | – |
| 11e | 2-Cl | CH | N | 7.48 ± 3.10 | 42.12 % | – |
| 11f | H | N | N | 0.46 ± 0.40 | 43.07 % | – |
| 15a | H | – | – | 0.38 ± 0.08 | 0.45 ± 0.02 | 0.84 |
| 15b | 2-CH3 | – | – | 0.39 ± 0.11 | 0.66 ± 0.16 | 0.59 |
| – | – | 1.49 ± 0.43f | 1.33 ± 0.55g | 1.12 | ||
| 15c | 3-CH3 | – | – | 0.54 ± 0.14 | 0.47 ± 0.17 | 1.14 |
| 15d | 4-CH3 | – | – | 24.77 ± 3.48 | 100.2 ± 22.09 | 0.24 |
| 15e | 3-OCH3 | – | – | 9.35 ± 2.32 | 20.34 ± 3.08 | 0.45 |
| 15f | 3-F | – | – | 1.04 ± 0.48 | 1.37 ± 0.74 | 0.75 |
| 15g | 3-Cl | – | – | 0.15 ± 0.02 | 1.01 ± 0.46 | 0.15 |
| 15h | 4-Cl | – | – | 1.95 ± 0.28 | 2.23 ± 0.81 | 0.87 |
| 15i | 2-Br | – | – | 0.42 ± 0.06 | 0.22 ± 0.04 | 1.99 |
| 15j | 3-Br | – | – | 0.39 ± 0.15 | 0.16 ± 0.04 | 2.44 |
| – | – | 1.25 ± 0.48f | 0.66 ± 0.22g | 1.89 | ||
| Tacrine | – | – | 0.02 ± 0.01 | 0.008 ± 0.004 | 2.50 | |
| Donepezil | – | – | 0.009 ± 0.001 | 1.57 ± 0.35 | 0.006 | |
AChE (EC 3.1.1.7) from electric eel.
BChE (EC 3.1.1.8) from horse serum.
Concentration required for 50% inhibition of ChEs, data were shown in mean ± SEM of triplicate independent experiments.
Inhibitory rate of the compounds under 100 μM on ChEs.
Selectivity index (SI)=AChE IC50/BChE IC50.
AChE (EC 3.1.1.7) from human.
BChE (EC 3.1.1.8) from human.
