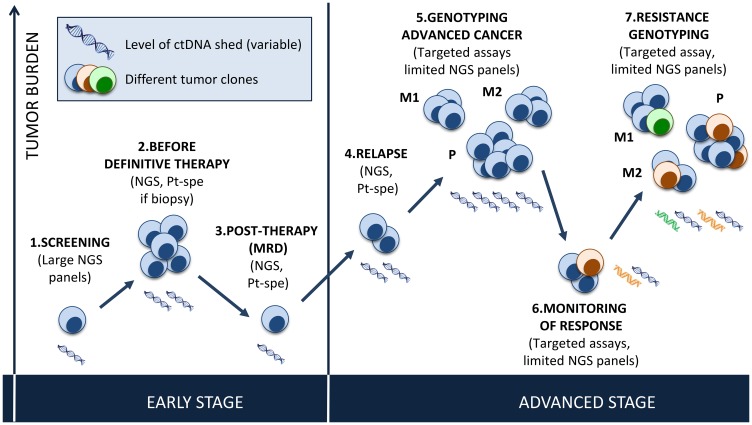
Figure 1. Potential use of plasma genotyping in cancer.
Early stage disease: Screening will require the use of large NGS panels, with both high sensitivity and perfect specificity. Before surgery, determination of tumor burden in plasma has the potential to help guide neo-adjuvant or adjuvant therapy and monitor response, using large panels or patient-specific assays based on the molecular profile of the tissue biopsy when available. After surgery, NGS (large gene panels or patient-specific assays) can detect MRD and guide adjuvant therapy (early detection) or detect relapse. Low tumor shed in plasma will be the main limitation to the integration of plasma genotyping in early stage disease. Advanced stage disease: At diagnosis, ctDNA can guide genotype-directed therapy (using targeted assays focusing on a predefined gene of interest (i. e. EGFR in NSCLC) or targeted NGS covering genes of interest). The variations in allelic fractions allow for monitoring of treatment response, which may be helpful for pharmacodynamics analyses in phase I studies. When acquired resistance to targeted therapies occurs, ctDNA can detect specific mechanisms of resistance (targeted assay like for EGFR T790M or targeted NGS), taking into consideration the different clones present within the primary tumor (P) and all metastatic sites (M1, M2), and guide treatment adjustments.