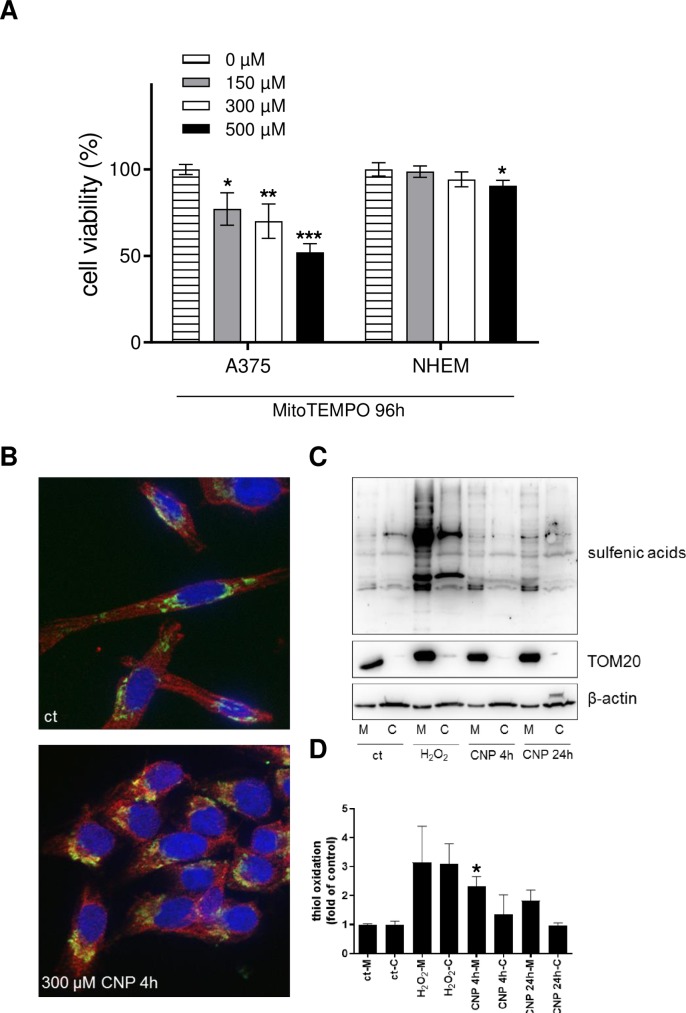
Fig 3. Mitochondrial ROS lower tumor cell viability and increase thiol oxidation.
A, MitoTEMPO, a mitochondrial superoxide scavenger that dismutates superoxide to hydrogen peroxide, decreased cell viability in a dose dependant manner after 96 h in A375, but not in NHEM. ANOVA with Dunnett´s post-hoc test was used for the determination of statistical significance. Data are means ± SEM, n = 3–6. *, p<0.05; **, p<0.01; ***, p<0.0001 compared to control. B, Nanoceria (300 μM) significantly increased mitochondrial thiol oxidation as indicated by immunofluorescence (cytochrome c: green; oxidized thiols: red) after 6 h of incubation (CNP 4 h, followed by addition of dimedone and additional 2 h of incubation). C, A375 cells were separated into cytosolic and mitochondrial fraction after mock-treatment, incubation with H2O2 or incubation with CNP. Western blot analysis revealed an increase in mitochondrial thiol oxidation compared to the cytosolic fraction. A representative Western blot of 4 individual experiments is shown (n = 4). D, Densitometric analysis of Western blot results of panel C. Thiol oxidation was determined relative to ß-actin and TOM20 signal for cytosol and mitochondria, respectively. The thiol oxidation was depicted as fold of control, which was set at 1. ANOVA with Tukey´s post-hoc test was used for the determination of statistical significance between the mitochondrial fractions of mock-treated and CNP-treated cells.