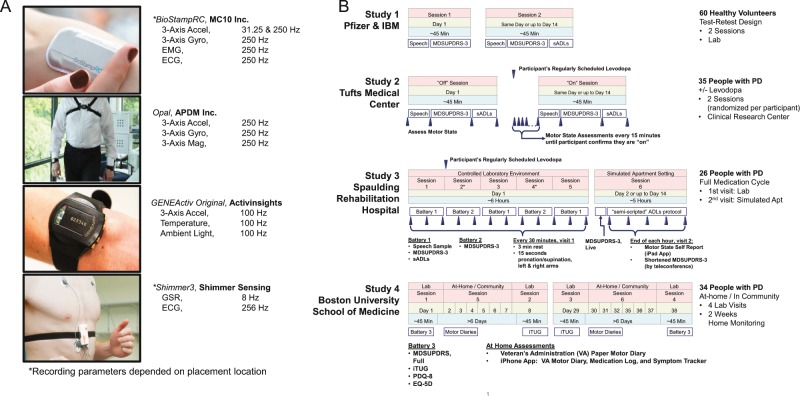
Fig. 1. Devices and study designs.
a Wearable devices and recording parameters that were utilized throughout the project. b In studies 1, 2, and 3, multiple administrations of the MDS-UPDRS part III, scripted activities of daily living (sADL’s), and speech tasks were performed. For participants with PD, sessions were timed to take place around PD participants’ regularly scheduled dose of levodopa, and we obtained both a live rating and three video ratings (from separate raters) of the MDS-UPDRS part III for each session. In addition, participants with PD self-reported their motor state multiple times throughout the laboratory sessions. Video raters were blinded to when the participant had taken their medications. In study 4, participants with PD completed 2 weeks of at-home monitoring with the ultimate goal of investigating the relationship between continuous estimates of motor state derived from wearable sensor data, participants’ self-report of their motor state, and the timing of levodopa. BioStampRC image used with permission from MC10. GENEActiv original image used with permission from Activinsights.