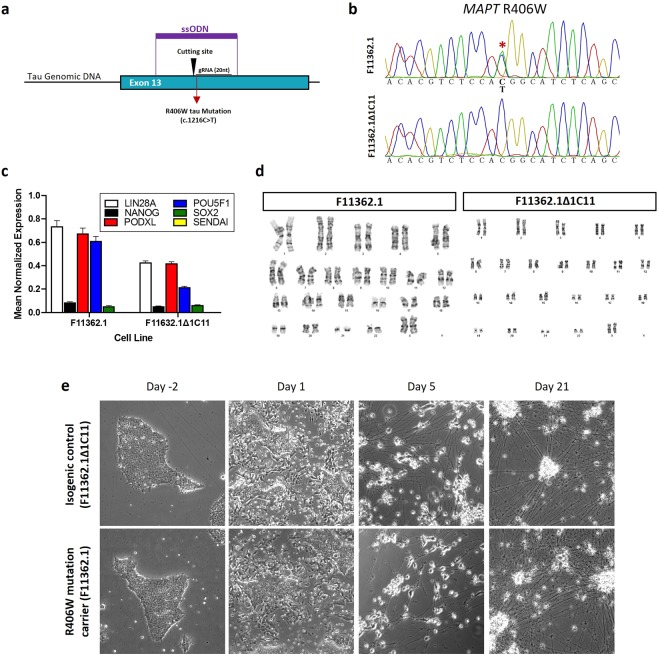
Figure 1.
Genetic correction and characterization of the patient-derived iPSC lines. (a) Schematic representation of the CRISPR/Cas9 gene engineering protocol used to correct the mutant allele (c.1216 C > T) in a human iPSC line carrying the R406W tau mutation (F11362.1) to generate an isogenic control iPSC line (F11362.1Δ1C11). (b) Sanger sequencing confirming the presence of a c.1216 C > T substitution in a single allele of exon 13 in the MAPT gene corresponding to the R406W tau mutation (F11362.1) that was corrected in the isogenic control iPSC line (F11362.1Δ1C11). (c) qPCR showing the relative expression of pluripotency markers from the embryonic stem cell lines. GAPDH was used as gene of reference (d) G-band karyotyping showing that both R406W tau mutation carrier (F11362.1) and CRISPR/Cas9-corrected control lines (F11362.1Δ1C11) display no chromosomal abnormalities. (e) Representative microscopy images (10x) showing the neuronal differentiation of both R406W mutant and isogenic control iPSC lines. Cells presented neuronal phenotype 21 days after starting the differentiation process. No differences were found in cell viability and neuronal differentiation between isogenic control (F11362.1Δ1C11) and R406W tau mutant (F11362.1) iPSC lines.