Highlights
-
•
Image invariance in the FFA increases from age seven to adulthood.
-
•
These results are confirmed by two independent ROI analyses.
-
•
Adaptation in the FFA relates to the ability to recognize a face in multiple images.
Keywords: Childhood, Development, Face processing, FFA, fMRI adaptation, Invariance
Abstract
Face recognition undergoes prolonged development from childhood to adulthood, thereby raising the question which neural underpinnings are driving this development. Here, we address the development of the neural foundation of the ability to recognize a face across naturally varying images. Fourteen children (ages, 7–10) and 14 adults (ages, 20–23) watched images of either the same or different faces in a functional magnetic resonance imaging adaptation paradigm. The same face was either presented in exact image repetitions or in varying images. Additionally, a subset of participants completed a behavioral task, in which they decided if the face in consecutively presented images belonged to the same person. Results revealed age-related increases in neural sensitivity to face identity in the fusiform face area. Importantly, ventral temporal face-selective regions exhibited more image-invariance – as indicated by stronger adaptation for different images of the same person – in adults compared to children. Crucially, the amount of adaptation to face identity across varying images was correlated with the ability to recognize individual faces in different images. These results suggest that the increase of image-invariance in face-selective regions might be related to the development of face recognition skills.
1. Introduction
In our daily life we encounter and recognize a vast number of faces. In most cases, we manage to recognize faces with great ease – even across varying contexts including differences in facial expression, lighting or viewpoint. How does this astonishing ability develop and what are its neural underpinnings?
It has been shown that the ability to discriminate and recognize faces undergoes prolonged development from childhood to adulthood (Germine et al., 2011; Weigelt et al., 2014). Similarly, on a neural level, the regions belonging to the network that subserves face recognition undergo development from childhood to adulthood (Cohen Kadosh et al., 2013a, 2013b, Golarai et al., 2007, Golarai et al., 2010, Golarai et al., 2017; Scherf et al., 2007). As such, a core component of the face-selective network, the fusiform face area (FFA; Kanwisher et al., 1997) exhibits developmental changes from childhood to adulthood: the FFA increases in size (Golarai et al., 2007; Peelen et al., 2009; Scherf et al., 2007), develops a stronger category-selectivity (Golarai et al., 2010; Peelen et al., 2009) and becomes increasingly sensitive to face identity (Natu et al., 2016).
Recently, behavioral studies have indicated that the ability to recognize a face in varying images might follow a prolonged trajectory from childhood to adulthood (Laurence and Mondloch, 2016; Neil et al., 2016) thus suggesting that the development of this ability might be linked to the overall increase in face recognition skills. Behavioral studies on the development of face recognition across varying images employed versions of a paradigm previously developed by Jenkins et al. (2011), in which participants are asked to sort images of faces containing natural within-person variability into piles according to their perceived identities. Adults divided 40 images of two identities into more identities than actually present with 7.5 identities on average (Jenkins et al., 2011). Notably, six- to 14-year-old children divided the images into even more identities with 14.5 identities on average (Neil et al., 2016).
Which neural underpinnings might be driving these differences between children and adults in image-invariant face recognition? The face-selective functional region FFA has been shown to process invariant aspects of faces crucial for identity recognition (Haxby et al., 2000; Rotshtein et al., 2004). To investigate image-invariance in the FFA and other functional regions, researchers have used fMRI adaptation (Grill-Spector and Malach, 2001), which is based on the mechanism that a certain brain region will show an adapted response to a repeated presentation of a certain stimulus feature, if this brain region is involved in processing this particular stimulus feature. This approach has proven to be a useful tool for studying neurodevelopmental processes (e.g., Natu et al., 2016; for a review see Nordt et al., 2016) as it does not require collecting behavioral responses thus avoiding possible confounds due to performance differences across groups (Poldrack, 2000). In case of image-invariance in the FFA, this suggests that the signal in the FFA should adapt to repeated presentations of the same face even when presented in different images. Using this approach, studies in adults have found that the FFA exhibits image-invariance up to a certain degree (Davies-Thompson et al., 2013), thereby suggesting that the development of the ability to recognize the same face across different images (Laurence and Mondloch, 2016; Neil et al., 2016) might be related to an increase of image-invariance in the FFA.
To investigate differences in image-invariance in the FFA between children and adults, in the present study we addressed the neurodevelopmental foundation of the ability to recognize the same face identity across naturally varying images. To this end, seven- to 10-year-old children and adults watched series of faces in a blocked fMRI adaptation paradigm (Grill-Spector and Malach, 2001; Nordt et al., 2016), comprising (i) the repeated presentation of the same face shown in the exact same image, (ii) the repeated presentation of the same face but in different images, and (iii) images of different faces. To obtain two independent measures of the development of invariance in face-selective regions, we employed both functional and structural approaches of defining face-selective regions in ventral temporal cortex (Weiner et al., 2014). Based on behavioral evidence (Laurence and Mondloch, 2016; Neil et al., 2016), we expected to find less adaptation for faces presented in varying images in children’s face-selective regions compared to adults. Thus, we hypothesized, that from childhood to adulthood face-selective regions exhibit greater invariance to image variability, that is to say, they become more proficient in recognizing the same face across varying images. Furthermore, we test if the amount of adaptation to the same face identity across different images is related to the behavioral ability of recognizing individual faces in different images.
2. Materials and methods
2.1. Participants
Child participants were recruited via newspaper articles and via a participant pool of families, who had already participated in other studies of the lab. Adult participants were recruited via announcements at the university. Data of 14 children (aged seven to 10 years, mean age = 8.36, SD = 0.81) and 14 adults (aged 20–23 years, mean age = 21.21, SD = 0.94) were included in the analyses. Three additional children were scanned but had to be excluded: Two datasets were excluded due to high head motion (motion > 3 mm) in the functional runs and one dataset had to be excluded due to technical error during data acquisition. Data of six adults were excluded due to the following reasons: one of them reported a psychiatric disorder (after being scanned) and data of five adults were discarded because their score on the Cambridge Face Memory Test was more than two standard deviations below the mean, thereby hinting at abnormal face processing (cut-off for prosopagnosia: 42.1 points or 58.47%, see Bowles et al., 2009).
As child participants are more difficult to recruit than adult participants, we included both right and left handed children and consecutively matched the adult sample to the child sample based on the parameters of handedness (Willems et al., 2010), gender and age range. Furthermore, we aimed at including similar amounts of data from children and adults to make sure that differences across groups are not due to differences in statistical power. After obtaining 14 data sets with high quality (indicated by low motion) in children, we matched the data of adult participants to that of children with regard to the factors handedness, gender, age range (four years in each age group) and number of participants across the age groups. By excluding additional five adult participants we achieved matching of groups for handedness (five left-handed participants in each age group), gender (4 female participants in each group) and age range (four years in each group). These datasets were excluded prior to any analyses (after checking for head motion values).
The remaining participants had normal or corrected vision and were free of neurological or psychiatric disorders. In each of the age groups four participants were left-handed (as indicated by self or – in case of child participants – parent report) and four participants were female. Adults’ performance on the Cambridge Face Memory Test (Duchaine and Nakayama, 2006) was on average 79.07% (SD = 8.87%, range: 62.5–94.44%) and children’s performance on the Cambridge Face Memory Test for Children (Croydon et al., 2014) was on average 81.68% (SD = 8.16%, range: 70.83–95.83%), demonstrating normal face recognition abilities in both groups.
Child participants were rewarded with a small present and a gift card and adult participants received course credit or monetary compensation for their participation. All participants or – in case of child participants – one of their caregivers gave informed written consent to participate in the study. Ethical approval was obtained from the local institutional review board.
2.2. Stimuli
2.2.1. Adaptation paradigm
Stimuli used in the adaptation paradigm consisted of 208 color images of the faces of 128 different individuals. Of those 128 individuals 112 were shown in only one image, while the remaining 16 individuals were depicted in six different images each. Half of the stimuli were taken from the internet and half of the stimuli were taken from two face databases including the PUT Face database (Kasiński et al., 2008) and the database “A lifespan database of adult facial stimuli” (Minear and Park, 2004). Faces taken from the internet were of sport team members of teams largely unknown in Germany (for examples of stimuli from the databases and from the Internet, see Supplementary material 1). All stimuli depicted faces of Caucasian adults. Both half of the stimuli from the internet and half of the stimuli from the database depicted male faces, while the other half depicted female faces. In all stimuli the face was seen in front view, with frontal gaze and with a neutral or friendly expression. Multiple images of the same individual differed only slightly from each other with small rotations of the head (approximately less than 5 ° from the front view). Furthermore, there were small changes of haircut, age and emotional expression across the six different images of the same individual. Images stemming from the internet contained a higher degree of variability compared to the images taken from the databases (for analyses of the effects of image variability see supplementary material 2). This was confirmed by the results of a simple rating task. In this task 12 adult participants, who did not take part in the fMRI adaptation paradigm, were given 16 small pieces of paper. On each piece six varying images of one face identity were printed in a row. Participants were asked to sort the pieces of paper (responding to the 16 identities) with regard to the variability of the six images of each face identity, from top indicating a high degree of variability to bottom indicating a low degree of variability. Results showed that images of faces from the internet were ranked as more variable compared to the images taken from the databases: 10 out of 12 participants sorted all 8 rows of images from the internet on the top 8 ranks, and the remaining 2 participants sorted 7 out of 8 image rows from the internet on the top eight ranks.
Image processing was done in Adobe Photoshop CS5 Extended. In a first step, the face (including the hair) was cropped. Subsequently, those images were scaled to a maximum image height of 207 pixels. The cropped face was then centered in a 250 × 250 pixels-sized square on a grey background. As the participants task was to press a button for blue-washed images, which occurred at a random time point within the block (see, Section 2.4.2 Task), we created “blue-washed” versions of all stimuli for the task using Photoshop. While these blue versions had the same grey background as the regular stimuli, the part of the image depicting the face had a slight transparent blue overlay. For examples of the stimuli, see Fig. 1. Scrambled versions of all images were made. The scrambled versions of blue-washed images were further edited with an additional slight transparent blue overlay (using Photoshop and the setting cyanotypie 215), to match the unscrambled blue images, as a behavioral pretest had shown behavioral differences in detecting blue images in scrambled and unscrambled images. We compared image contrast for images belonging to different experimental conditions using the graycomatrix() and the graycoprops() functions in MATLAB. These functions provide a measure of the intensity contrast between a pixel and its neighbor over the whole image. Importantly, the analysis revealed that the images used in the same-face-different-images condition and the images in the different-faces condition did not differ significantly from each other, t(206) = −0.005, p = .996. Note that the images used in the same-face-same-image condition were drawn from the same image set as the ones for the different-faces condition, as images were randomly selected from the pool of images for each participant for those two conditions.
Fig. 1.
Example for the stimuli used in the adaptation paradigm. Rows represent example stimuli of each of the three main conditions. Top row: different-faces condition. Middle row: same-face-different-images condition, bottom row: same-face-same-image condition.
2.2.2. Localizer
Stimuli used in the functional localizer comprised images of faces and objects. The face stimuli consisted of 24 neutral looking faces of 12 men and 12 women with frontal gaze, which were taken from the Karolinska Directed Emotional Faces Database (Lundqvist et al., 1998). The object stimuli were images of 24 objects from the objects database used in Konkle and Oliva (2012). All objects were chosen to have a real-world size similar to the size of faces.
2.3. General procedure
One to seven days before the MRI-measurements (mean number of days = 3.6, SD = 2.2) children participated in a mock-scanner training. During the mock-scanner training children were familiarized with the environment of an MRI scanner in a stepwise manner and completed short practice runs. Furthermore, they performed a practice version of the experimental task using a separate stimulus set and completed the Cambridge Face Memory Test for Children (Croydon et al., 2014). Since adult participants did not take part in the mock-scanner training, they completed the Cambridge Face Memory Test (Duchaine and Nakayama, 2006) prior to the MRI measurement. During the structural sequence of the MRI-measurement participants watched short cartoon movie clips. The MRI-measurement lasted approximately 30–45 min.
Participants were invited to perform a behavioral task (see same/different-face-decision task) on a separate visit to the lab, which took place a couple of weeks or months after the MRI session.
2.4. Scanning
2.4.1. Structural and functional imaging data acquisition
Structural and functional MR images were obtained from each participant. Imaging was performed on a 3-Tesla Philips Achieva 3.2 MRI scanner using a 32-channel head coil. Functional images for both the adaptation paradigm and the functional localizer were acquired using a blood oxygen level dependent (BOLD) sensitive T2*-weighted sequence with the following parameters: number of slices = 33, TR = 2000 ms, TE = 30 ms, voxel size = 3 × 3 × 3 mm, gap between slices: 0.4 mm, flip angle: 90, field of view: 240 mm × 240 mm. Each run of the adaptation paradigm consisted of 112 volumes and each localizer run consisted of 66 volumes. The slice scan order was ascending interleaved. Four dummy scans were acquired at the beginning of each functional run before the onset of the paradigm to allow for T1 equilibrium and were not included into the analysis. High-resolution, T1-weighted, structural images were acquired containing 220 sagittal slices (FOV: 240 × 188 × 220 mm, voxel size 1 × 1 × 1 mm). Stimuli were presented using the VisuaStim Digital (Resonance Technology, Inc., California USA) goggle system. The paradigm was run on a Dell Latitude E5540 (Round Rock, USA) machine with 4GB of RAM and an Intel Core i5-4300U 1.9–2.5 GHz CPU using MATLAB (version 2008a, Mathworks) and psychtoolbox (version 3.0.9, Brainard, 1997).
2.4.2. Task during the fMRI measurements
To sustain attention throughout the whole experiment, to minimize differences with regard to attention demands between experimental conditions, and between attentional allocation between children and adults, we used a task that was equally suited for child and adult participants: The participants’ task was to press a button for blue-washed images. There was one image with a blue overlay in each block, occurring at a random time point within the block. Also in the scrambled images of the baseline trials there was always one blue-washed image, which was generated from scrambling the blue-washed images. The task was the same for the adaptation and the localizer runs. For the localizer runs the button had to be pressed for blue faces, blue objects or blue squares.
2.4.3. Functional localizer
The functional localizer consisted of eleven blocks, which followed a palindromic design. Of the eleven blocks the first, sixth (middle) and last block were blocks of fixation in which participants saw squares in different colors. The remaining blocks were alternating blocks of face and object stimuli. In each block six images (or in case of the fixation six colored squares) were presented for 1750 ms each, followed by 250 ms of grey screen. Image size was 250 × 250 pixels. As in the experimental paradigm, a blue-washed version of the stimuli of the localizer was created, so that participants were able to perform the same task as during the adaptation experiment (see Section 2.4.2 Task).
2.4.4. Adaptation paradigm
A blocked design comprising four runs was applied. Each run consisted of 12 face blocks and of seven baseline blocks. In each face block six images were presented for 1750 ms each and were separated from the following image by 250 ms of blank gray screen. All images were presented on a light grey background. There were three main conditions: (1) Same-face-same-image condition: In this condition a single image of one face was presented repeatedly during the block. (2) Same-face-different-images condition: In this condition different images of the same person were presented. (3) Different-faces condition: In this condition images of different faces were presented. The three conditions occurred equally often within runs. Additionally, the position of the images on the screen was varied: While in half of the blocks the position of the image was kept constant in the center of the screen, in the other half of the blocks, the position of the image was randomly shifted one third of the stimulus size to the left, right, up- or downwards. Furthermore, the position was varied in half of the blocks of each condition. For example in one run, the position of the images was varied in two of four same-face- same-image blocks and was kept constant in the other two same-face-same image blocks (for analyses of the effects of the position shifts of the images, see supplementary material 3. Half of the blocks comprised male and the other half female faces. The gender of the face was kept constant within blocks. Blocks were randomized within runs with the constraint that not more than two blocks of each of the three main conditions followed each other. To rule out order effects, images were randomly assigned to the blocks and runs for each participant. No participant saw the same face in more than one block. Baseline blocks were presented at the beginning and after every second face block and consisted of three images. Images in the baseline blocks were scrambled versions of the face images and were randomly selected from the pool of images. Stimulus presentation was equal to that in face blocks. Before and after each face and baseline block a 500 × 500 pixel-sized black square was presented for 1750 ms followed by 250 ms of blank screen to create visual boundaries between blocks. We maximized the power of the adaptation paradigm compared to previous studies. For instance in (Scherf et al., 2011) a total of 24 time points of measurement were acquired per experimental condition (resulting from: six blocks per condition, blocks including 12 images and lasting 12 s, a TR of three seconds). In contrast, in the present study we acquired 96 time points of measurement per condition (resulting from: 16 blocks per condition, blocks including 6 images and lasting 12 s, a TR of two seconds).
2.5. Behavioral paradigm after the fMRI measurement (same/different-face-decision task)
To obtain a measure on the ability to recognize the same face in varying images, participants completed a behavioral task containing the images from the adaptation paradigm. As this task was administered on a separate day, only a subset of participants participated (including eight children and eight adults). In this behavioral task participants were presented with 88 trials, each consisting of two images. In half of the trials both images depicted the same face, while in the other half the two images depicted different faces. Of the trials showing the same face, half of the trials showed the same face presented in the same image and the other half showed the same face but presented in different images. The participants’ task was to indicate whether the same face or different faces were presented in the trial. After presentation of the second face, a grey screen was shown and participants were instructed to provide an answer via button press. Whenever participants gave a response, the next trial began. Participants were instructed to answer as accurately as possible. Participants were instructed to press the “1″ key on the keyboard if the two faces belonged to the same person and the “0″ key if the faces belonged to different persons. To facilitate identification of the keys, yellow and green stickers were placed on the keys. The images were presented on a grey background one after another for 750 ms each and were separated by a 250 ms interstimulus interval. The presentation time of stimuli was chosen to be shorter compared to the adaptation paradigm to prevent ceiling effects. Stimuli were randomly drawn from the pool of stimuli used in the adaptation paradigm (see Section 2.1 Stimuli). Children completed a short practice version prior to the actual task. This practice version contained faces of cartoon characters for each of the three possible conditions (same-face-same-image, same-face-different-images, different-faces) to make sure that children had understood the task. The task was run on a MacBook Pro (OS X El Capitan, 2Ghz Intel Core i7) with a screen size of 15″ using MATLAB (version R2016a, Mathworks) and the Psychtoolbox (version 3.0.12, Brainard, 1997).
2.6. Analysis
2.6.1. fMRI data preprocessing
Preprocessing of fMRI data was done using BrainVoyager QX (Version 2.8.4.2645, BrainInnovation, Maastricht, The Netherlands) and included temporal filtering including linear trend removal, 3D motion correction and slice time correction. For temporal filtering a high-pass filter (GLM-Fourier, 2 sines/cosines) was applied. 3D motion correction was performed using trilinear/sync interpolation, including the BrainVoyager QX intrasession alignment option with the volume closest to the T1-weighted anatomical scan taken as reference. Slice scan time correction was done using cubic spline interpolation.
2.6.2. Processing of structural images
We used published surface-based methods in FreeSurfer (http://surfer.nmr.mgh.harvard.edu, version 5.3.0) to reconstruct the cortical surfaces of the T1-weighted images. The details of this procedure have been described elsewhere (Dale et al., 1999; Fischl et al., 1999). The automatic reconstruction steps included skull stripping, gray and white matter segmentation as well as reconstruction and inflation of the cortical surface. These processing steps were performed for each participant individually. After preprocessing, each individual’s segmentation was quality controlled slice by slice and inaccuracies for the automatic steps were corrected by manual editing if necessary. After anatomical partitions were defined in FreeSurfer (see Section 2.7.1 Anatomical approach), they were imported into BrainVoyager and were manually transformed into ACPC and Talairach space to run the statistical analysis. Surface reconstruction was not possible in the dataset of one child. Therefore, analyses based on the surface reconstruction are based on a sample of 27 participants (14 adults, 13 children).
2.6.3. Statistical analysis
Statistical analyses were performed using R, version 3.4.0 (http://www.R-project.org). For all analyses, linear parametric methods were used. Statistical comparisons included paired t-tests or repeated measures ANOVAs (rmANOVAs). Testing was two-tailed with an α-level of 0.05. For post-hoc t-tests we report Holm’s correction for multiple comparisons.
2.7. Region of interest definition
We used both an anatomical and a functional approach for defining our regions of interest for two reasons. First, we did not obtain a similar amount of data in children and adults of the functional localizer, as it was administered at the end of scanning sessions and some children were tired of scanning at this point. To be able to include data from participants without functional localizer runs, we defined ROIs both functionally and anatomically. Second, defining ROIs both anatomically and functionally allowed us two obtain two independent measures for our data
2.7.1. Anatomical approach
Anatomical partitions in the VTC were individually defined on the surface reconstructions of each participant similar to the procedure applied in Golarai et al. (2017). We individually defined six partitions in the VTC of each hemisphere in each participant’s native space. The partitions were defined as a patch on VTC with the following boundaries: The lateral boundary in each hemisphere was defined as a line along the lateral boundary of the occipitotemporal sulcus (OTS) and the medial boundary was defined at the lateral boundary of the collateral sulcus (CoS). The posterior end was defined by the posterior transverse collateral sulcus (ptCoS). The anterior border was placed approximately at 60% from the ptCos to the anterior pole of the temporal lobe. The patch was subdivided into six partitions by two horizontal lines at 20% and 40% from the ptCos to the anterior pole of the temporal lobe and by a vertical line which bisected the midfusiform sulcus (MFS). An example of the partitions can be seen in Fig. 3f. It has been shown that face-selective regions can be predicted based on the location of the MFS (Weiner et al., 2014). Weiner et al. (2014) demonstrated that in 83 ± 7% of the cases, the face-selective region FFA-2 lies lateral to the anterior tip of the MFS and can be predicted based on this anatomical landmark quite reliably. Based on this finding we predicted that the middle lateral patch of our VTC divisions would include FFA-2 (see Fig. 3f). The face-selective region FFA-1 can be predicted based on the location of posterolateral tip of the MFS, and consequently lies within the posterior lateral patch in our partitions. Note however, that while the coupling between the location of MFS and FFA-2 is quite strong, the coupling between the location of the MFS and FFA-1 is weaker (Weiner et al., 2014).
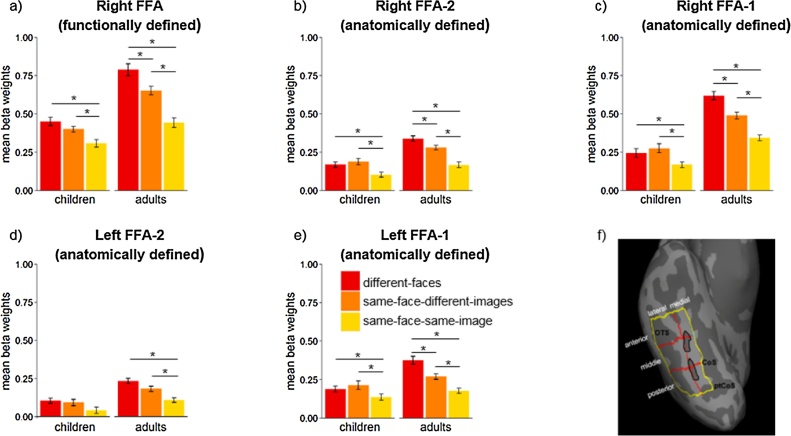
Fig. 3.
Responses in functionally and anatomically defined ROIs. The top row shows ROIs in the right hemisphere and the bottom row ROIs in the left hemisphere. Red: different-faces condition; orange: same-face-different-images condition; yellow: same-face-same-image condition. (a) Functionally defined right FFA. (b) Anatomically defined right FFA-2. (c) Anatomically defined right FFA-1. (d) Anatomically defined left FFA-2. (e) Anatomically defined left FFA-1. (f) Example of the partitions on VTC shown on the inflated surface of one representative participant. OTS: Occipitotemporal sulcus. CoS: Collateral sulcus. ptCos: posterior transverse collateral sulcus. Circled in black: Midfusiform sulcus (MFS). Error bars represent the standard error of the mean (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
2.7.1.1. Validation of the anatomical approach
Testing for the regional specificity assuming that lateral, but not medial patches contain face-selective regions, we conducted a 2 × 3 rmANOVA in each hemisphere with mean activation as the dependent variable and adaptation condition (different-faces, same-face-different-images, same-face-same-image) and location (lateral, medial; each including posterior and middle patches) as independent variables. Besides the main effect of condition (right hemisphere: F(2,52) = 21.18, p < .001, = 0.07, left hemisphere: F(2,52) = 10.67, p < .001, = 0.04), we found a main effect of location (right hemisphere: F(1,26) = 71.99, p < .001, = 0.40, left hemisphere: F(1,26) = 33.69, p < .001, = 0.24), and an interaction between condition and location (right hemisphere: F(1.53, 39.83) = 8.13, p = .002, = 0.003, left hemisphere: F(1.36, 35.23) = 5.62, p = .015, = 0.004). While lateral patches in both hemispheres were positively activated in all three conditions (all p < .001), activity in medial patches was not significantly different from zero (all p > .05), or below zero (for the same-face-same-image-condition in right hemisphere, t(26) = −2.69, p = .012), thus providing evidence for the regional specificity assuming that lateral, but not medial patches of our VTC divisions contain face-selective regions.
2.7.2. Functional approach
We obtained 28 localizer runs in adults and 13 runs in children. Four runs (2 of children and 2 of adults) were discarded due to high motion. Localizer data of one participant including two further runs had to be discarded due to unsuccessful surface reconstruction in this participant’s structural images. For the functional definition of the face selective region in VTC we masked the VTC by using the outer boundary of the anatomical partitions and defined the FFA within this patch as the supra-threshold voxels under the contrast faces > objects using the functional localizer.
2.8. Controlling for quality of BOLD data between children and adults
To address the concern that general differences between age groups might influence results of developmental brain imaging studies (for a more detailed description of this issue, see Golarai et al., 2017), we analyzed several factors concerning data quality such as amount of data, motion, axial and coronal brain-to-coil distance (Golarai et al., 2017). Furthermore, we compared age groups regarding their task performance during the scanner to assure that both groups were paying attention to the images. Finally, in whole-brain analyses we tested if the adaptation paradigm activated the face network.
2.8.1. Matching of motion of imaging data and amount of data to achieve equal power across groups
For each functional run maximum absolute values of each of the six motion parameters (x-, y-, z-translation and x-, y-, z-rotation) were computed. Runs with motion exceeding 3 mm or 3 ° in any of the parameters were discarded from the analysis (4 runs in children and 2 runs in adults). The mean of the maximum absolute values of the six parameters was computed for each participant and each run. These values were used to compare children’s motion to that of adults. After discarding runs of the adaptation paradigm due to high motion, 48 runs remained in the child sample, indicating that each child contributed on average 3.4 usable functional runs of the adaptation paradigm. To achieve comparable power in the adaptation paradigm between the groups as to assure that any difference between groups would not attributable to differences in power, 48 runs were randomly selected from the 54 runs from the adult sample and were included in the analysis
After discarding runs with motion > 3 mm, overall motion of the functional runs from the adaptation paradigm was matched between children and adults (see, Supplementary Fig. 4a, t(93.3) = 0.24, p = .81), with children moving on average M = 0.44 mm or degree (SD = 0.31) and adults moving on average M = 0.42 mm or degree (SD = 0.34). In the localizer paradigm, motion was comparable across the groups as well (t(12.45) = 0.21, p = .84), with children moving on average M = 0.28 mm or degree (SD = 0.30) and adults moving on average M = 0.25 mm or degree (SD = 0.26).
2.8.2. Brain-to-coil distance (axial and coronal)
To control for possible differences in the BOLD signal in VTC due to differences in head size, we computed both axial and coronal brain-to-coil distance (Golarai et al., 2017). Therefore, we estimated both the axial and coronal distance from the cortex to the skull. The axial brain to coil distance was assessed by measuring the distance between the occipital pole and the outer edge of the skull to obtain a measure for the proximity of the occipital cortex to the coil. The coronal brain to coil distance was estimated by measuring the shortest distance between the outer edges of the left and right ear canal in each participant, thereby obtaining an indirect measure. Estimation of coronal and axial distances was performed by two persons independently. Subsequently, the mean of those two estimates was computed.
Axial brain-to-coil distance differed significantly between children and adults (t(19.13) = 4.74, p < .001), with a mean distance of M = 7.96 mm (SD = 1.91) in children and M = 10.82 mm (SD = 2.02) in adults (see Supplementary Fig. 4b), as found similarly in (Golarai et al., 2017). There was no difference between children and adults in coronal brain-to-coil distance (t(26) = 0.36, p = .72), with a mean difference of M = 134.79 mm (SD = 5.88) in children and M = 133.96 mm (SD = 6.28) in adults (see Supplementary Fig. 4c).
2.8.3. Task performance during the fMRI measurement
To assure that both groups paid attention to the images, participants were instructed to indicate the detection of blue-washed images by button press.
In the adaptation paradigm, overall performance was highly accurate for both children (hit rate: M = 98.10%, SD = 2.5) and adults (hit rate: M = 97.54%, SD = 6.62). To assess if there were specific attentional differences between the three adaptation conditions, we conducted a 2 × 3 rmANOVA including hit rate as a dependent variable and group (children, adults) and adaptation condition (same-face-same-image, same-face-different-images, different-faces; not including the scrambled condition) as independent variables. The rmANOVA revealed a main effect of group, F(1,26) = 4.78, p = .038, = 0.16, indicating higher detection performance in adults (M = 99.70, SD = 0.76) compared to children (M = 98.07, SD = 2.7), but no significant effect of condition, F(2,52) = 0.64, p = .54, nor an interaction between group and condition, F(2,52) = 0.18, p = .84, showing that participants paid high amounts of attention during the three main conditions. Similarly, accuracy during the functional localizer paradigm was high in both groups and did not differ significantly (t(19) = 0.0, p = 1, note, that not all children completed the localizer runs, see above), with adults achieving a mean hit rate of 98.05% (SD = 2.94) and children achieving a hit rate of 98.05% (SD = 3.58).
2.8.4. Whole brain analyses
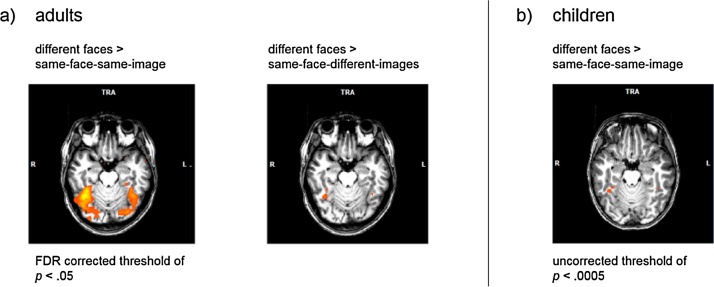
Testing for the specificity of the adaptation paradigm, we conducted whole-brain analyses in both children and adults. We found that the adaptation paradigm quite specifically activated the face-system In adults, several clusters in the VTC and surrounding visual cortex emerged for the different-faces > same-face-same-image contrast, hence showing adapted responses to the repetition of a (facial) image. Among other regions, the clusters were found in right and left fusiform gyrus. More importantly, only two regions – one of them presumably right FFA – emerged from the same-face-different-images > same-face-same-image contrast. Five clusters emerged for the different-faces > same-face-different images contrast including a region in the fusiform gyrus in both the right and left hemisphere (see Fig. 2, Table 1). In children, no significant clusters emerged at a false-discovery corrected (FDR) threshold (see Table 1). Using an uncorrected statistical threshold of p < .0005, the different-faces > same-face-same-image contrast revealed four significant clusters, the two largest clusters presubably corresponding to right and left FFA (see Table 2).
Fig. 2.
Results of the whole-brain analysis for the adaptation paradigm for adults and children. (a) Whole brain analysis in adults for the different faces > same-face-same-image contrast (left) and for the different-faces > same-face-different-image contrast (right), presented at an FDR corrected threshold of p < .05. (b) Whole-brain analysis in children for the different-faces > same-face-same-image contrast at an uncorrected threshold of p <.0005. In children no significant clusters were observed for the other contrasts (see Table 2).
Table 1.
Significant clusters of the whole brain analysis using an FDR corrected threshold.
| group | condition | hemi-sphere | number of voxels | TAL coordinates of significant cluster |
Brodmann Area | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| adults | DiffDiff > SameSame | RH | 25253 | 30.28 | −69.94 | −8.27 | Fusiform (BA 37) |
| 890 | 21.96 | −83.13 | 24.03 | BA 19 | |||
| 432 | 24.25 | −3.00 | −12.10 | Amygdala (BA53) | |||
| 157 | 7.50 | −42.86 | 49.94 | BA 7 | |||
| 99 | 1.86 | 57.98 | −5.53 | BA 10 | |||
| 34 | 32.65 | −42.79 | 47.65 | BA7 | |||
| 29 | 33.59 | −7.17 | −26.45 | Parahippocampus(BA 36) | |||
| LH | 17158 | −33.77 | −71.57 | −8.03 | BA 19 | ||
| 53 | −25.79 | −31.00 | −15.89 | Fusiform (BA 37) | |||
| 48 | −29.02 | −84.48 | 22.19 | BA 19 | |||
| 47 | −9.23 | 59.55 | −8.15 | BA 10 | |||
| 39 | −27.08 | 18.21 | −26.31 | BA 38 | |||
| 31 | −27.71 | −60.55 | −8.32 | Fusiform BA 37) | |||
| 24 | −18.17 | −4.62 | −13.71 | Amygdala | |||
| SameDiff > SameSame | RH | 74 | 39.88 | −50.16 | −15.49 | Fusiform (BA 37) | |
| 16 | 41.12 | −76.00 | −5.00 | BA 19 | |||
| DiffDiff > SameDiff | RH | 131 | 35.23 | −50.69 | −17.95 | Outside defined BAs, probably in the fusiform gyrus | |
| 87 | 33.61 | −77.43 | −0.82 | BA 19 | |||
| 14 | 33.43 | −39.86 | −17.00 | Fusiform (BA 37) | |||
| LH | 22 | −37.55 | −71.18 | −13.91 | Outside defined BAs | ||
| 17 | −37.18 | −46.47 | −17.88 | Fusiform (37) | |||
| children | DiffDiff > SameSame | none | |||||
| SameDiff > SameSame | none | ||||||
| DiffDiff > SameDiff | none | ||||||
Note. Clusters are organized according to hemisphere and size. Minimum cluster size was 10 voxels. Diff: different-faces condition; same-diff: same-face-different-image condition; same-same: same-face-same-image condition. Brodmann Areas are defined according to the BioImage Suite developed by Yale University (http://sprout022.sprout.yale.edu/mni2tal/mni2tal.html).
Table 2.
Significant clusters of the whole brain analysis in children using an uncorrected threshold of p < .0005.
| group | condition | hemi-sphere | number of voxels | TAL coordinates of significant cluster |
Brodmann Area | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| children | DiffDiff > SameSame | RH | 35 | 37.49 | −43.97 | −17.20 | Fusiform (BA 37) |
| 18 | 31.22 | −38.61 | −17.00 | Fusiform (BA 37) | |||
| LH | 20 | −38.20 | −40.45 | −18.25 | Fusiform (BA 37) | ||
| 10 | −42.30 | −9.90 | −23.00 | BA 20 | |||
| SameDiff > SameSame | none | ||||||
| DiffDiff > SameDiff | none | ||||||
Note. Clusters are organized according to hemisphere and size. Minimum cluster size was 10 voxels. Diff: different-faces condition; same-diff: same-face-different-image condition; same-same: same-face-same-image condition. Brodmann Areas are defined according to the BioImage Suite developed by Yale University (http://sprout022.sprout.yale.edu/mni2tal/mni2tal.html).
3. Results
In the present study, we aimed at investigating if the image-invariance in the FFA increases from age seven to adulthood. To this end, 14 children and 14 adults watched images of faces in an fMRI adaptation block design including three main conditions comprising (i) the presentation of different faces, (ii) the presentation of the same face in different images, and (iii) the repeated presentation of the same face in the same image. To elucidate if changes in image-invariance in the FFA were related to behavior, a subset of participants completed a behavioral task outside the scanner in which two faces were presented consecutively and participants had to decide, if the two faces belonged to the same person.
3.1. Do both children and adults exhibit neural sensitivity to face identity in the FFA? Is image-invariance in the FFA comparable across the age groups?
For analysis of the fMRI adaptation paradigm (see Fig. 1), we employed two approaches to define regions of interest (ROIs) in VTC. In the first approach we used a standard independent functional localizer (faces > objects) to define face-selective regions in VTC (see section Methods, Section 2.7.2 Region of interest definition, Functional approach). In the following, we will refer to this ROI as functionally defined FFA. In the second approach, we used anatomical landmarks to define partitions in VTC in each hemisphere of each participant (following Golarai et al., 2017); see Section 2.7.1 Region of interest definition, Anatomical approach and Fig. 3f). With respect to the ROIs defined by anatomical landmarks, according to Weiner et al. (2014), face-selective regions in the fusiform gyrus can be predicted based on the location of the midfusiform sulcus (MFS). Based on this finding we assume that in our partitions on VTC, the middle lateral patch overlaps with FFA-2, and the posterior lateral patch overlaps with FFA-1. In the following we refer to the anatomically defined patch in middle lateral VTC overlapping with FFA-2 as anatomically defined FFA-2, and to the patch in posterior lateral VTC overlapping with FFA-1 as anatomically defined FFA-1.
We tested in each of the previously defined ROIs, if children and adults exhibited adaptation to face identity, and more importantly, if the amount of adaptation differed across the age groups, thereby indicating changes in image-invariance in the FFA. To this end we ran separate 3 × 2 rmANOVAs including the factors adaptation condition (different-faces, same-face-different-images, same-face-same-image) and age group (children, adults) in each ROI in each hemisphere. Differences in adaptation were tested for the right and left hemisphere separately based on findings showing that face processing relies more heavily on the right compared to the left hemisphere (although not exclusively, see Behrmann and Plaut, 2015).
3.1.1. Right hemisphere
Activity in the functionally defined FFA varied for the three adaptation conditions (main effect of adaptation condition, F(2,50) = 36.89, p < .001, = 0.13). Furthermore, overall activity was higher in adults compared to children (main effect of age group, F(1,25) = 5.93, p = .02, = 0.18). Most importantly, adults and children exhibited different levels of adaptation to the same face presented in different images (adaptation condition × age group interaction, F(2,50) = 6.01, p = .005, = 0.02, see Fig. 3a, Table 3). Both children (t(12) = 3.70, p = .009), and adults (t(13) = −6.40, p < .001), showed adaptation to repetition of a face identity, as indicated by an adapted response for the presentation of the same face in the same image relative to the presentation of different faces (same-face-same-image < different-faces). Moreover, both groups exhibited an adapted response for the same face presented in the same image, compared to the same face presented in different images (same-face-same-image < same-face-different-images, children: t(12) = −3.23, p = .014; adults: t(13) = −5.96, p < .001). Crucially, only adults, (t(13) = −2.75, p < .017), but not children (t(12) = 1.60, p = .154), showed an adapted response when the same face was presented in different images (same-face-different-images < different-images), thereby indicating that the FFA in adults exhibits a greater degree of image-invariance comparison to the FFA in children. Notably, this pattern of results was replicated when ROIs in the right hemisphere were anatomically defined (see Table 3 for an overview and Supplementary Material 5 for a detailed description of all results). Both anatomically defined FFA-1 and FFA-2 showed a significant interaction between adaptation condition and age group (both Fs ≥ 4.68, ps ≤ .014, Fig. 3b,c) which was driven by significant adaptation to the same face presented in different images in adults, but not in children.
Table 3.
Overview on effects in functionally and anatomically defined ROIs.
| Region of interest | Effect of adaptation condition | Effect of age | Age × adaptation condition interaction |
|---|---|---|---|
| Right functionally defined FFA | F(2,50) = 36.89, p < .001 | F(1,25) = 5.93, p = .02 | F(2,50) = 6.01, p = .005 |
| Right anatomically defined FFA-2 | F(2,50) = 25.94, p < .001 | F(1,25) = 5.84, p = .023 | F(2,50) = 4.68, p = .014 |
| Right anatomically defined FFA-1 | F(2,50) = 28.87, p < .001 | F(1,25) = 5.47, p = .028 | F(2,50) = 9.42, p < .001 |
| Left functionally defined FFA | – | – | – |
| Left anatomically defined FFA-2 | F(2,50) = 13.99, p < .001 | ns | ns |
| Left anatomically defined FFA-1 | F(2,50) = 17.59, p < .001 | ns | F(2,50) = 6.73, p = .003 |
In sum, results of both functionally and anatomically defined ROIs in the right hemisphere consistently suggest that while both children and adults show neural sensitivity to face identity in ventral temporal face-selective regions, these regions exhibit a smaller degree of image-invariance in children compared to those in adults.
3.1.2. Left hemisphere
Results from the functionally defined left FFA are lacking, as the analysis of the standard localizer did not reveal any significant clusters in the left hemisphere for the contrast faces > objects in the location of the FFA. In the left anatomically defined FFA-2 activity was – much as in the right hemisphere – significantly affected by the adaptation condition (main effect of adaptation condition, F(2,50) = 13.99, p < .001, = 0.06), but contrary to the right hemisphere, activity neither differed between age groups (no main effect of age, F(1,25) = 2.37, p = 0.14), nor was there an interaction between age group and adaptation condition (no age group × adaptation condition interaction, F(2,50) = 1.48, p < .24, Fig. 3d). T-tests comparing the adaptation conditions in data of children and adults combined revealed that the signal adapted when the same face was presented in the same image relative to different image (face-same-image < different-faces, t(26) = −5.18, p < .001), and also showed that the signal adapted for the same face in the same image compared to the same face in different images (same-face-same-image < same-face-different-images, t(26) = −3.38, p = .005). In contrast, there was no adaptation for the same face in different images (same-face-different-images < different-images, t(26) = −1.75, p = .092).
While results from the anatomically defined FFA-1 in the left hemisphere revealed overall similar activity between age groups (no effect of age group, F(1,25) = 1.46, p = .24), the remaining pattern of results was as in the right hemisphere. Most importantly, the effect of the adaptation conditions varied across children and adults (adaptation condition × age group interaction, F(2,50) = 6.73, p = .003, = 0.02, see Fig. 3e), with only adults exhibiting adaptation to face identity despite varying images of a face (same-face-different-image < different-faces, children: t(12) = 0.70, p = .50, adults: t(13) = 3.07, p = .009).
In sum, results from the left hemisphere deviate from those in the right hemisphere, as in the anatomically defined FFA-2 in the left hemisphere no interaction between adaptation condition and age group was found. In the anatomically defined FFA-1 in the left hemisphere however, once more the pattern of results suggests that – consistent with the ROIS in the right hemisphere – both children and adults show neural sensitivity to face identity in face-selective regions, with a smaller degree of image-invariance of face-selective regions in children compared to adults.
3.2. Prolonged maturation of the ability to recognize a face across different images
To obtain a measure for the behavioral ability to recognize a face in multiple images we tested a subset of participants (8 children, 8 adults), with a same/different-face-decision task. In this task participants had to decide, if a face in two consecutively presented images belonged to the same person. In half of the trials the two faces belonged to the same person and in the other half faces belonged to different persons. Of the trials showing the same face/person, half of the trials showed the same face in the same image, while half of the trials showed the same face in different images.
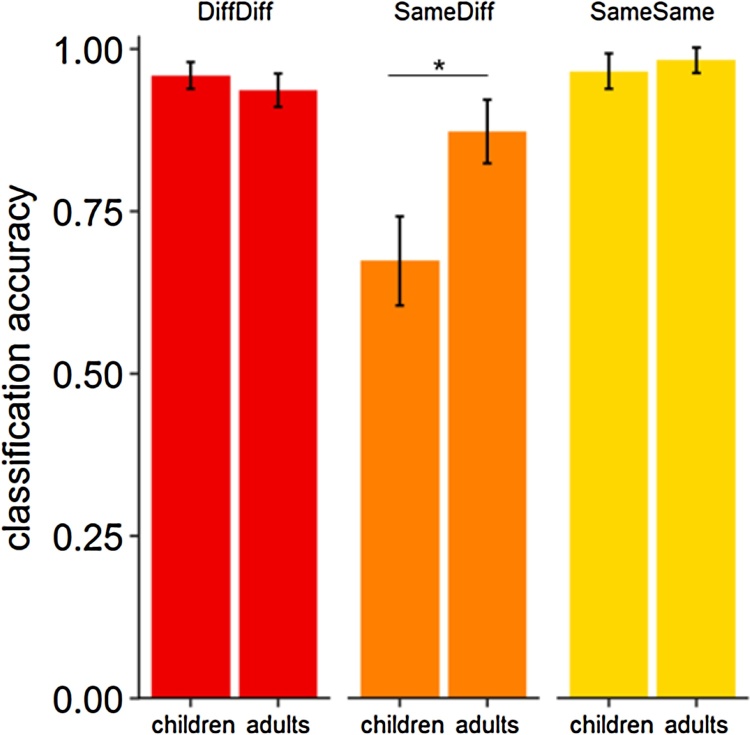
We found that with regard to accuracy, children performed worse compared to adults, when a face had to be recognized across different images, while performance was matched in the other two conditions. This was revealed by a 2 × 2 rmANOVA including accuracy as a dependent variable and the factors age group (children, adults) and face identity (same, different) as independent variables. Performance in this task differed for trials including the same face and for trials including different faces (main effect of face identity, F(1,14) = 15.00, p = .002, = 0.44). Furthermore, overall performance was higher in adults compared to children (main effect of age group, F(1,14) = 14.44, p = .002, = 0.21). Crucially, there was differential development for the two conditions (face identity × age group interaction, F(1,14) = 11.52, p = .004, = 0.38, see Fig. 4). While performance in the different-faces condition was similar across groups (t(14) = −0.79 p = .44), it differed for the same-face condition, (t(14) = 6.91, p < .001). As half of the trials of the same-face condition included the exact repetition of an image and the other half consisted of the same face presented in different images, we compared performance for these different trial types. Performance across groups did not differ for trials in which the same face was presented in the same image (t(14) = 0.92, p = .37). Importantly, it differed for the trials in which the same identity was presented in different images, and participants had to decide if the face in the image belonged to the same person (t(14) = 5.79, p < .001). That is, children selectively performed worse in the condition, in which a facial identity had to be recognized across differing images (see Fig. 4). To control for ceiling effects, we compared performance in each group to the maximum performance level in each condition. While children’s performance was at ceiling in the different-faces condition (M = 0.96, SD = 0.05), t(7) = −2.25, p = .06), it was not at ceiling in the same-face-same-image condition (M = 0.97, SD = 0.04), t(7) = 2.39, p = .048). In the adults, performance was at ceiling in the same-face-same-image condition (M = 0.98, SD = 0.03), t(7) = −1.43, p = 20), however, it differed significantly from ceiling in the different-faces condition (M = 0.94, SD = 0.06), t(7) = −2.99, p = .02).
Fig. 4.
Performance in the behavioral task (same/different-face-decision task). Percent correct performance is shown for trials in which different faces were presented (on the left) and same faces were presented (middle and right). Same faces trials comprised either the same face presented in different images (middle) or the same face presented in the same image (on the right). Please note that in 50% of the trials participants saw two different faces and in 50% of the trials participants saw the same face to prevent a response bias. Accordingly, the SameDiff and SameSame trials account for 25% of the trials each. Error bars represent 95% confidence intervals.
3.3. The ability to recognize a face across different images correlates with the amount of adaptation in ventral temporal face-selective regions for repetitions of an individual face shown in different images
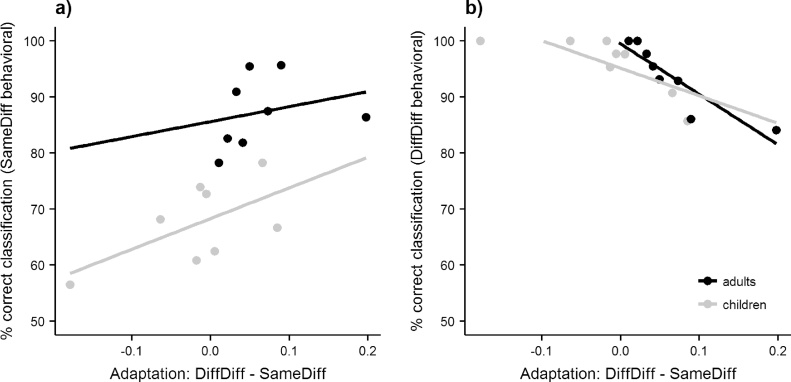
We tested whether the behavioral performance in the same/different-face-decision task was related to the amount of neural adaptation in face-selective regions. Indeed, we found that participants, who were better at deciding, if two faces belonged to the same person, showed more adaptation to the same face presented in different images in face-selective regions. This was demonstrated by a positive correlation between the amount of neural adaptation for the same-face-different-images condition (relative to the different-faces condition) in right anatomically defined FFA-2 and the behavioral performance in the same-face-different-images condition of the same/different-face-decision task, r = 0.65, p = .007 (see Fig. 5a). When the correlation was controlled for the effect of age, it remained marginally significant, rp = 0.51, p = .054, indicating that this effect was not merely driven by general age-related changes. Furthermore, we found a negative correlation between the performance in the different-images condition of the same/different-face decision task and the amount of neural adaptation for the same-face-different-images condition (relative to the different-faces condition) in right anatomically defined FFA-2, r = −.79, p < .001 (see Fig. 5b). When the correlation was controlled for the effect of age, it remained significant, rp = −0.81, p < .001. A similar correlation was found in the functionally defined rFFA: Performance in the different-images condition of the same/different-face decision task correlated negatively with the amount of neuronal adaptation for the same-face-different-images condition (relative to the different-faces condition), rp = −.70, p = .004 (corrected for the effect of age). In combination, these correlations suggest that the stronger the neural adaptation effect, i.e. the bigger the difference between the different-images and the same-face-different-images condition and hence the more image-invariance there is, the more a participant is inclined to answer “same person” in the behavioral task. In case of the same-face-different-images condition this is beneficial (correct), in case of the different-faces condition this is detrimental (incorrect).
Fig. 5.
Correlation between the amount of neural adaptation and behavior. This figure depicts the correlation in the same-face-different-images condition in right anatomically defined FFA-2 with two behavioral measures. (a) Correlation between the amount of adaptation in the same-face-different-images condition (relative to the different-faces condition) and the behavioral performance in the same-face-different-images condition. (b) Correlation between the amount of adaptation in the same-face-different-images condition (relative to the different-faces condition) and the behavioral performance in the different-faces condition. gray dots: children; black dots: adults.
Behavioral performance in the same-face-different-image condition of the same/different-face-decision task correlated with overall BOLD activity in the different-faces condition, r = .74. p = .001, which persisted also when correcting for age, rp = 0.54, p = .037. No other significant correlations with the overall BOLD activity were observed in any of the other ROIs.
In sum, this suggests that the ability to recognize a face across different images correlates with the amount of adaptation in both functionally and anatomically defined right FFA. Specifically, the amount of adaptation (same-face-different-images < different images) in functionally defined rFFA and anatomically defined rFFA-2 was negatively correlated with performance in a task in which one has to decide if images of two different individuals depict the same face or not. That is, greater amounts of adaptation were associated with the tendency to decide that two images depict the same individual, when this was not the case. Furthermore, the amount of adaptation (same-face-different-images < different images) in anatomically defined rFFA-2 correlated with performance in the condition, in which participants were supposed to recognize the same individual across two different images. In other words, participants, who were better at deciding, that images of two faces belonged to the same individual, showed more adaptation to the same face presented in different images.
4. Discussion
The present study addressed the development of the neural foundation of the ability to recognize faces in varying images. Results of this study indicate, first of all, that both seven- to 10-year-old children and adults exhibit neural sensitivity to face identity (as measured by fMRI adaptation) in face-selective regions, in line with previous results (Natu et al., 2016). Second and most importantly, our results revealed that face-selective regions in adults exhibit a greater degree of image-invariance for face identity. Third, our results suggest that the neural sensitivity towards individual faces presented in varying images is correlated with the ability to recognize a face across varying images.
The finding of neural sensitivity to face identity in both children and adults in the present study is coherent with recent findings of neural sensitivity to face identity (Natu et al., 2016), but contrasts with findings from earlier studies, which did not show neural sensitivity to face identity in the form of fMRI adaptation in the FFA in six- to 10-year-old children (Scherf et al., 2011). The discrepancies might be explained by differences in statistical power of the study designs: In our study we had 96 time points per experimental condition compared to 24 time points of measurement per condition in Scherf et al. (2011). The result indicating that neural sensitivity to faces is present in childhood, but is higher in adulthood is consistent with results from behavioral studies suggesting that children perform well on face recognition tasks, but nevertheless show improvements of this ability until adulthood (Germine et al., 2011; Weigelt et al., 2014).
Our results suggest that face-selective regions in adults show a greater degree of invariance towards image variability compared to face-selective regions in childhood. In face-selective regions in adults, but not in children, we observed adaptation to the same face presented in varying images. Several studies in adults have addressed image-invariance of ventral temporal face-selective regions with mixed results, presumably depending on the type of variation (e.g., viewpoint changes, changes in lighting, changes in size) and the amount of variation (e.g., small vs large rotations) across images. For instance, an early study showed stronger invariance in posterior fusiform gyrus to changes in position and size compared to changes in viewpoint and illumination (Grill-Spector et al., 1999). Ewbank and Andrews (2008) reported a release from adaptation, i.e. no invariance, for viewpoint changes larger than 4° in unfamiliar faces in the FFA suggesting that for unfamiliar faces images-invariance is only achieved for small rotations in viewpoint. Results from the present study as well as from Weibert et al. (2016) are coherent with this finding, as both studies reported image-invariance in the FFA for unfamiliar faces for stimuli comprising only very small viewpoint changes.
A similar increase in invariance with age has been reported for view invariance in object recognition (Nishimura et al., 2015). In the study by Nishimura and colleagues five- to10-year-old children, 11-17-year-old adolescents and adults were presented with images of objects changing in size and viewpoint in an fMRI adaptation paradigm. Results showed that the amount of invariance for different views of objects (as indicated by the amount of adaptation) within lateral occipital complex (LOC) increased from childhood to adulthood, while the invariance to variations in object size did not change across age groups. Furthermore, the amount of adaptation correlated positively with recognition performance for objects after a change of viewpoint.
Converging evidence from research on face processing comes from a study using parametrically varying morph faces (Natu et al., 2016). In the study by Natu et al. (2016) children and adults were presented with faces that varied in their similarity by creating morphs between two different faces. The resulting faces ranged from being very similar to very dissimilar depending on the amount each face contributed to the morph face. Natu and colleagues fit linear models on each participant’s activation in face-selective regions, and compared the slopes of these models for the face morphs varying in similarity. This analysis revealed that slopes were larger in face-selective regions in adults compared to children, showing that adults’ face-selective regions were more sensitive to face dissimilarity.
Taken together, results from the present study as well as the studies by Natu et al. and Nishimura et al. suggest that the difference between the neural response to the repetition of the same stimulus identity and different stimulus identities becomes more pronounced from childhood to adulthood in face- and object-selective regions. This greater signal difference might allow a more precise differentiation between the same identity presented in varying conditions (such as viewpoint) and processing of different identities, which is also reflected in increasing behavioral performance with age in all three studies.
In the present study, the difference between adults and children in invariance of ventral temporal face-selective regions varied for the right and left hemisphere. While in the right hemisphere a greater amount of invariance in adults compared to children was observed in all three ROIs (right functionally defined FFA, anatomically defined FFA-1 and FFA-2), in the left hemisphere this effect was present only in the anatomically defined FFA-1 (note however, that no functionally defined left FFA could be identified). This stronger development in the right compared to the left hemisphere might be related to the stronger (albeit not complete) lateralization of face processing to the right hemisphere, which has been proposed to be associated with the increasing tuning of left ventral temporal regions to words (Behrmann and Plaut, 2015).
We found that the ability to recognize a face in varying images, was correlated with the amount of adaptation for faces presented in varying images in right FFA-2 (anatomically defined), as well as in the functionally defined right FFA. In the behavioral task participants were to indicate if the faces presented in two images, shown one after each other, belonged to the same person (or not). We found that children selectively performed worse compared to adults in the condition of the task, in which two varying images of the same face were presented. Children, in comparison to adults, decided more often that the presented face belonged to different identities, thus showing lesser degree of image-invariance on a behavioral level. This finding is in line with initial results from behavioral studies suggesting a prolonged development in a task, in which multiple images of two faces have to be sorted according to their perceived identity and in which six- to seven-year-old children divided the images into even more identities than adults (Neil et al., 2016). The present results provide a link between these behavioral findings and the development on a neural basis.
5. Limitations and future directions
As face recognition relies on a network of brain regions, future neurodevelopmental studies – ideally using longitudinal designs and larger sample sizes – might compare and relate the development of image-invariance in the FFA to that in other brain regions, such as the occipital face area (see also, Cohen Kadosh et al., 2013b), as well as face patches in the anterior temporal lobe, which have been suggested to be involved in face recognition (Elbich and Scherf, 2017), and invariant face recognition in particular (Anzellotti et al., 2014; Nestor et al., 2011). Ultimately, investigating the face network as a whole will help to gain a more coherent picture of face processing and its development from childhood to adulthood.
6. Conclusions
In conclusion, results of the present study suggest that from childhood to adulthood face-selective regions become more invariant towards image-variability and that this increase might be related to the development of face recognition skills.
Funding sources
This work was supported by a scholarship of the German National Academic Foundation [Studienstiftung des deutschen Volkes awarded to M.N.]; a grant from the German Research Foundation [DFG WE5802/1-1 to S.W.] and a grant from the Daimler and Benz Foundation [to S.W.]. The authors declare no competing financial interests.
Acknowledgements
We thank Sabine Bierstedt, Astrid Hönekopp, Tobias W. Meissner, Luzie Mount, Helen Prüfer, Katharina Sommer and Sophia Terwiel for assistance in data collection. We also thank Burkhard Mädler from PHILIPS Germany for scientific support.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2018.04.005.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Neil L., Cappagli G., Karaminis T., Jenkins R., Pellicano E. Recognizing the same face in different contexts: testing within-person face recognition in typical development and in autism. J. Exp. Child Psychol. 2016;143:139–153. doi: 10.1016/j.jecp.2015.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzellotti S., Fairhall S.L., Caramazza A. Decoding representations of face identity that are tolerant to rotation. Cereb. Cortex. 2014;24:1988–1995. doi: 10.1093/cercor/bht046. [DOI] [PubMed] [Google Scholar]
- Behrmann M., Plaut D.C. A vision of graded hemispheric specialization. Ann. N. Y. Acad. Sci. 2015;1359:30–46. doi: 10.1111/nyas.12833. [DOI] [PubMed] [Google Scholar]
- Brainard D.H. The psychophysics toolbox. Spat. Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- Bowles D.C., McKone E., Dawek A., Duchaine B., Palermo R., Schmalzl L. Diagnosing prosopagnosia: effects of ageing, sex and participant-stmulus ethnic match on the Cambridge Face Memory Test and Cambridge Face perception Test. Cogn. Neuropsychol. 2009;26:344–423. doi: 10.1080/02643290903343149. [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh K., Johnson M.H., Dick F., Cohen Kadosh R., Blakemore S.-J. Effects of age, task performance, and structural brain development on face processing. Cereb. Cortex. 2013;23:1630–1642. doi: 10.1093/cercor/bhs150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh K., Johnson M.H., Henson R.N.A., Dick F., Blakemore S.-J. Differential face-network adaptation in children, adolescents and adults. NeuroImage. 2013;69:11–20. doi: 10.1016/j.neuroimage.2012.11.060. [DOI] [PubMed] [Google Scholar]
- Croydon A., Pimperton H., Ewing L., Duchaine B.C., Pellicano E. The Cambridge face memory test for children (CFMT-C): a new tool for measuring face recognition skills in childhood. Neuropsychologia. 2014;62:60–67. doi: 10.1016/j.neuropsychologia.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis: I. segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Davies-Thompson J., Newling K., Andrews T.J. Image-invariant responses in face-selective regions do not explain the perceptual advantage for familiar face recognition. Cereb. Cortex. 2013;23:370–377. doi: 10.1093/cercor/bhs024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchaine B., Nakayama K. The Cambridge face memory test: results for neurologically intact individuals and an investigation of its validity using inverted face stimuli and prosopagnosic participants. Neuropsychologia. 2006;44:576–585. doi: 10.1016/j.neuropsychologia.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Elbich D.B., Scherf S. Beyond the FFA: brain-behavior correspondences in face recognition abilities. NeuroImage. 2017;147:409–422. doi: 10.1016/j.neuroimage.2016.12.042. [DOI] [PubMed] [Google Scholar]
- Ewbank M.P., Andrews T.J. Differential sensitivity for viewpoint between familiar and unfamiliar faces in human visual cortex. NeuroImage. 2008;(40):1857–1870. doi: 10.1016/j.neuroimage.2008.01.049. [DOI] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Germine L.T., Duchaine B., Nakayama K. Where cognitive development and aging meet: face learning ability peaks after age 30. Cognition. 2011;118:201–210. doi: 10.1016/j.cognition.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Golarai G., Ghahremani D.G., Whitfield-Gabrieli S., Reiss A., Eberhardt J.L., Gabrieli J.D.E., Grill-Spector K. Differential development of high-level visual cortex correlates with category-specific recognition memory. Nat. Neurosci. 2007:512–522. doi: 10.1038/nn1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golarai G., Liberman A., Grill-Spector K. Experience shapes the development of neural substrates of face processing in human ventral temporal cortex. Cereb. Cortex. 2017 doi: 10.1093/cercor/bhv314. bhv314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golarai G., Liberman A., Yoon J.M.D., Grill-Spector K. Differential development of the ventral visual cortex extends through adolescence. Front. Hum. Neurosci. 2010;3 doi: 10.3389/neuro.09.080.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K., Malach R. fMR-adaptation: a tool for studying the functional properties of human cortical neurons. Acta Psychol. (Amst.) 2001;107:293–321. doi: 10.1016/s0001-6918(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K., Kushnir T., Edelman S., Avidan G., Itzchak Y., Malach R. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron. 1999;24:187–203. doi: 10.1016/s0896-6273(00)80832-6. [DOI] [PubMed] [Google Scholar]
- Haxby J.V., Hoffman E.A., Gobbini M.I. The distributed human neural system for face perception. Trends Cogn. Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Jenkins R., White D., Van Montfort X., Mike Burton A. Variability in photos of the same face. Cognition. 2011;121:313–323. doi: 10.1016/j.cognition.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Kanwisher N., McDermott J., Chun M.M. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 1997;1(7):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasiński A., Florek A., Schmidt A. The PUT face database. Image Process. Commun. 2008;13:59–64. [Google Scholar]
- Konkle T., Oliva A. A real-world size organization of object responses in occipitotemporal cortex. Neuron. 2012;74:1114–1124. doi: 10.1016/j.neuron.2012.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence S., Mondloch C.J. That’s my teacher! Children’s ability to recognize personally familiar and unfamiliar faces improves with age. J. Exp. Child. Psychol. 2016;143:123–138. doi: 10.1016/j.jecp.2015.09.030. [DOI] [PubMed] [Google Scholar]
- Lundqvist D., Flykt A., Öhman A. Department of Clinical Neuroscience, Psychology section, Karolinska Institutet; Stockholm, Sweden: 1998. The Karolinska Directed Emotional Faces KDEF. [Google Scholar]
- Minear M., Park D.C. A lifespan database of adult facial stimuli. Behav. Res. Methods Instrum. Comput. 2004;36:630–633. doi: 10.3758/bf03206543. [DOI] [PubMed] [Google Scholar]
- Natu V.S., Barnett M.A., Hartley J., Gomez J., Stigliani A., Grill-Spector K. Development of neural sensitivity to face identity correlates with perceptual discriminability. J. Neurosci. 2016;36:10893–10907. doi: 10.1523/JNEUROSCI.1886-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor A., Plaut D.C., Behrmann M. Unraveling the distributed neural code of facial identity through spatiotemporal pattern analysis. Proc. Natl. Acad. Sci. 2011;108:9998–10003. doi: 10.1073/pnas.1102433108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Scherf K.S., Zachariou V., Tarr M.J., Behrmann M. Size precedes view: developmental emergence of invariant object representations in lateral occipital complex. J. Cogn. Neurosci. 2015;27:474–491. doi: 10.1162/jocn_a_00720. [DOI] [PubMed] [Google Scholar]
- Nordt M., Hoehl S., Weigelt S. The use of repetition suppression paradigms in developmental cognitive neuroscience. Cortex. 2016;80:61–75. doi: 10.1016/j.cortex.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Peelen M.V., Glaser B., Vuilleumier P., Eliez S. Differential development of selectivity for faces and bodies in the fusiform gyrus. Dev. Sci. 2009;12:F16–F25. doi: 10.1111/j.1467-7687.2009.00916.x. [DOI] [PubMed] [Google Scholar]
- Poldrack R.A. Imaging brain plasticity: conceptual and methodological issues— a theoretical review. NeuroImage. 2000;12:1–13. doi: 10.1006/nimg.2000.0596. [DOI] [PubMed] [Google Scholar]
- Rotshtein P., Henson R.N.A., Treves A., Driver J., Dolan R.J. Morphing marilyn into maggie dissociates physical and identity face representations in the brain. Nat. Neurosci. 2004;8:107. doi: 10.1038/nn1370. [DOI] [PubMed] [Google Scholar]
- Scherf K.S., Behrmann M., Humphreys K., Luna B. Visual category-selectivity for faces, places and objects emerges along different developmental trajectories. Dev. Sci. 2007;10:15–30. doi: 10.1111/j.1467-7687.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- Scherf K.S., Luna B., Avidan G., Behrmann M. “What” precedes “which”: developmental neural tuning in face- and place-related cortex. Cereb. Cortex. 2011;21:1963–1980. doi: 10.1093/cercor/bhq269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibert K., Harris R.J., Mitchell A., Byrne H., Young A.W., Andrews T.J. An image-invariant neural response to familiar faces in the human medial temporal lobe. Cortex. 2016;84:34–42. doi: 10.1016/j.cortex.2016.08.014. [DOI] [PubMed] [Google Scholar]
- Weigelt S., Koldewyn K., Dilks D.D., Balas B., McKone E., Kanwisher N. Domain-specific development of face memory but not face perception. Dev. Sci. 2014;17:47–58. doi: 10.1111/desc.12089. [DOI] [PubMed] [Google Scholar]
- Weiner K.S., Golarai G., Caspers J., Chuapoco M.R., Mohlberg H., Zilles K., Amunts K., Grill-Spector K. The mid-fusiform sulcus: a landmark identifying both cytoarchitectonic and functional divisions of human ventral temporal cortex. NeuroImage. 2014;84:453–465. doi: 10.1016/j.neuroimage.2013.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems R.M., Peelen M.V., Hagoort P. Cerebral lateralization of face-selective and body-selective visual areas depends on handedness. Cereb. Cortex. 2010;20:1719–1725. doi: 10.1093/cercor/bhp234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.