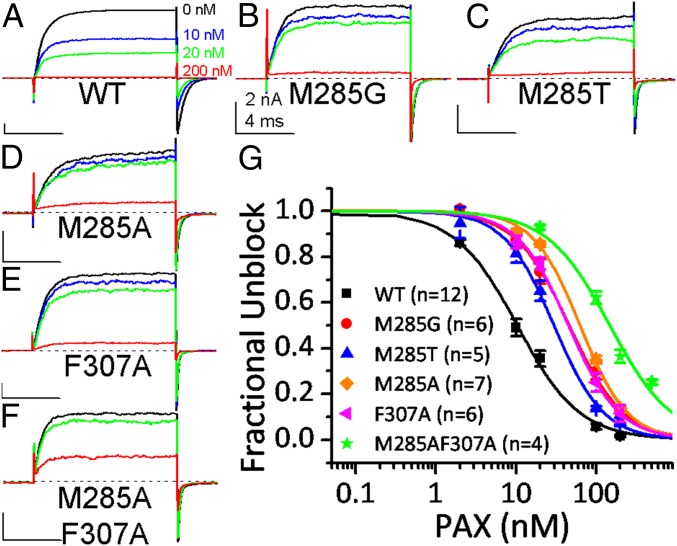
Fig. 4.
Effect of binding crevice mutations on the PAX sensitivity of BK channels. WT or mutated BK channels were held at 0 mV with 10 μM [Ca2+]in when exposed to various concentrations of PAX. BK current was evoked by a 10-ms step to 160 mV every 5 s to determine the fraction of unblocked channels. (A–F) The sample traces of WT (A), M285G (B), M285T (C), M285A (D), F307A (E), and M285AF307A (F) BK channels in 0 nM (black), 10 nM (blue), 20 nM (green), and 200 nM (red) PAX. The dotted line marks the 0-current level. (G) Dose–response curves of BK inhibition by PAX. Hill equation fit results (solid lines) are IC50 = 10.4 ± 0.6 nM, n = 1.1 ± 0.07 for WT (black symbol and line); IC50 = 46.3 ± 2.5 nM, n = 1.3 ± 0.07 for M285G (red symbol and line); IC50 = 29.9 ± 1.4 nM, n = 1.4 ± 0.07 for M285T (blue symbol and line); IC50 = 63.3 ± 3.3 nM, n = 1.5 ± 0.09 for M285A (orange symbol and line); IC50 = 45.4 ± 1.9 nM, n = 1.4 ± 0.05 for F307A (magenta symbol and line); and IC50 = 148.8 ± 9.1 nM, n = 1.1 ± 0.09 for M285AF307A (green symbol and line). The number of experiments contributing to each dose–response curve is in parentheses.