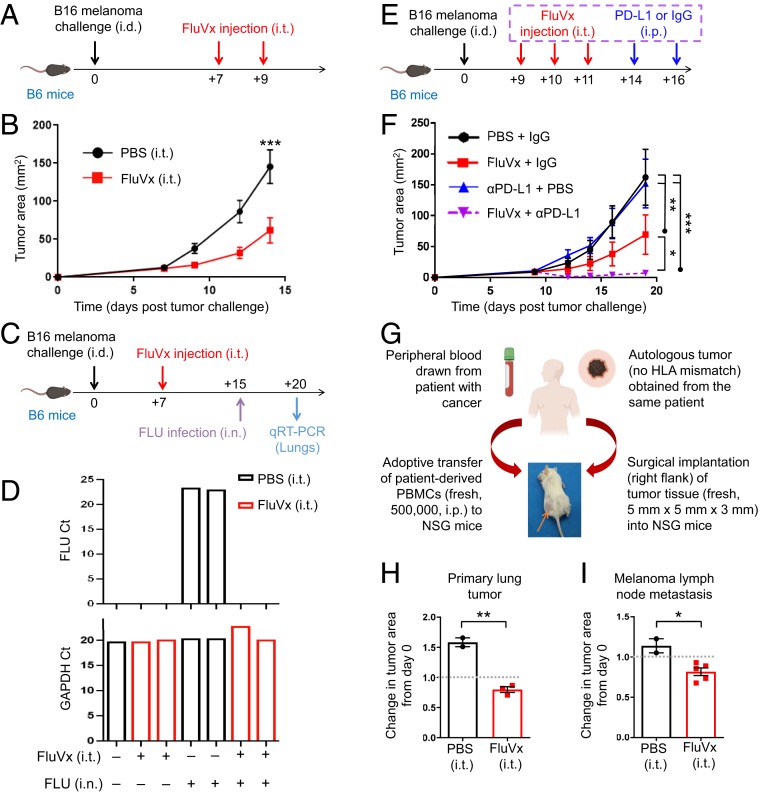
Fig. 3.
Intratumoral unadjuvanted seasonal influenza vaccine administration reduces tumor growth, augments checkpoint blockade immunotherapy, and protects against active influenza virus lung infection. (A) Experimental design. Unadjuvanted seasonal influenza vaccine (FluVx): FluVx1. n = 9 mice/group. Data are representative of at least 2 independent experiments with similar results. (B) Tumor growth curves from experiment described in A. (C) Experimental design. FluVx: FluVx1. (D) Bar graphs showing count threshold (Ct) of active influenza virus (FLU) or GAPDH control qRT-PCR transcripts from experiment described in C. (E) Experimental design. FluVx: FluVx1. n = 4 to 5 mice/group. Data are representative of at least 2 independent experiments with similar results. (F) Tumor growth curves from experiment described in E. (G) Schematic describing development of the AIR-PDX model in which NSG mice receive adoptive transfer of fresh patient-derived human peripheral blood mononuclear cells (PBMCs; 500,000 cells) and surgically implanted tumor sections (∼5 mm × 5 mm × 3 mm) from the same (i.e., autologous) patient. FluVx: FluVx1. n = 2 to 5 mice/group. (H) Bar graphs showing change in area of a primary lung tumor (day 13 area/day 0 area) from experiment described in G. Dotted line corresponds to day 0 (first treatment day). (I) Bar graphs showing change in area of a melanoma lymph node metastasis (day 16 area/day 0 area) from experiment described in G. Dotted line corresponds to day 0 (first treatment day). *P < 0.05, **P < 0.01, ***P < 0.001 [2-way ANOVA with Bonferroni correction (B) or Tukey correction (F), 2-tailed Student t test (H and I)]. Error bars: mean ± SEM. i.d., intradermal; i.t., intratumoral; i.p., intraperitoneal; i.n., intranasal. IgG, control isotype antibody; αPD-L1, PD-L1 blocking antibody.