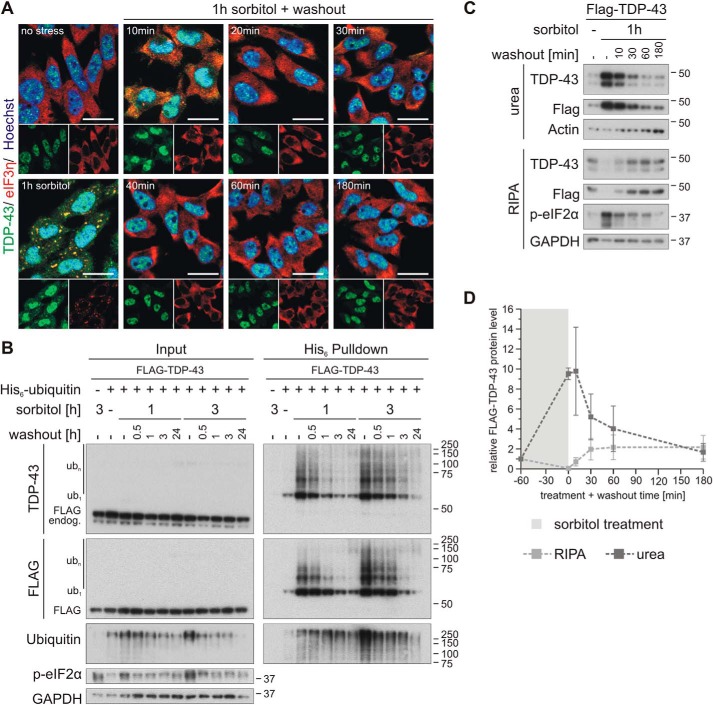
Figure 5.
Osmotic stress–induced TDP-43 ubiquitylation and insolubility are rapidly reversible. A, HEK293E cells were stressed with 0.4 m sorbitol for 1 h followed by washout with fresh medium for 10–180 min, as indicated. The fixed cells were subjected to immunofluorescence analysis with specific antibodies against endogenous TDP-43 (green) and the SG marker eIF3η (red). Cell nuclei were counterstained with Hoechst 33342 (blue). Scale bars, 20 μm. B, cells transfected with FLAG-TDP-43 and His6-ubiquitin (+) or His6-control vector (−) were exposed to osmotic stress with sorbitol for 1 or 3 h or left untreated (−). After a washout for 0.5–24 h, as indicated, the cells were lysed with urea buffer, and ubiquitylated proteins were isolated by His6 pulldown with Ni-NTA–agarose. Total protein (Input) and eluates (His6 Pulldown) were analyzed by Western blotting with antibodies detecting TDP-43, FLAG, ubiquitin, phospho-eIF2α, and GAPDH. C, after treatment with sorbitol for 1 h, HEK293E cells expressing FLAG-TDP-43 were washed out for 10–180 min and were subjected to sequential extraction. The RIPA-soluble and -insoluble urea protein lysates were analyzed by Western blotting with antibodies against TDP-43, FLAG, and phospho-eIF2α and GAPDH and actin as loading controls. D, quantification of FLAG-TDP-43 protein levels normalized to actin from three independent experiments as in C. Band intensities were normalized to nonstressed control conditions (−). The data represent the mean ± S.E. (error bars).