Figure 6.
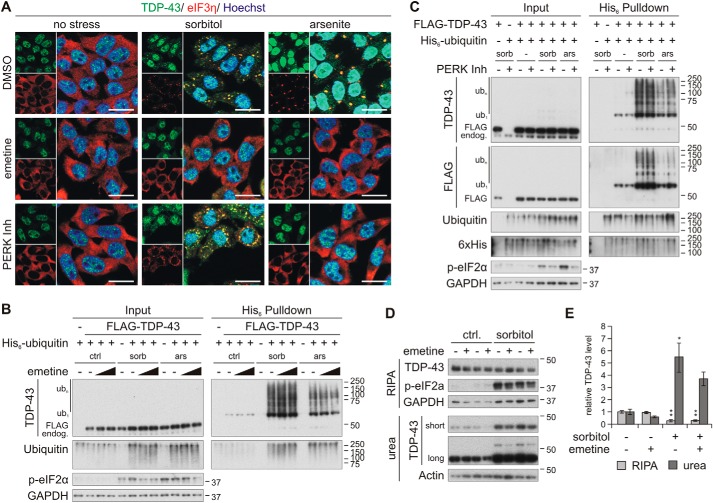
Osmotic stress-induced ubiquitylation and insolubility of TDP-43 are not dependent on its translocation into stress granules. A, HEK293E cells were pretreated with 25 μg/ml emetine or the PERK inhibitor GSK2606414 (PERK Inh, 10 μm) and stressed with sorbitol or arsenite. The fixed cells were immunostained with rabbit-anti-TDP-43 (green) and mouse-anti-eIF3η (red). Cell nuclei were counterstained with Hoechst 33342 (blue). Scale bars, 20 μm. B and C, FLAG-TDP-43 (+), His6-ubiquitin (+), or empty control vectors (−) were expressed in cells that were pretreated with 10 or 25 μg/ml emetine (B) or 10 μm PERK inhibitor (C), followed by exposure to sorbitol (sorb) or arsenite (ars) in the presence of the inhibitors, as indicated. His6-ubiquitin–conjugated proteins were purified from urea cell lysates with Ni-NTA–agarose and were subjected to Western blot analysis with antibodies detecting TDP-43, FLAG, ubiquitin, His6, phospho-eIF2α, and the loading control GAPDH. D, solubility analysis of emetine-pretreated HEK293E cells that were exposed to control medium (−) or stressed with sorbitol (+). RIPA-soluble and -insoluble urea fractions were analyzed with Western blotting using TDP-43, phospho-eIF2a, and GAPDH or actin antibodies. E, quantification of TDP-43 protein levels normalized to actin from three independent experiments as in D. Band intensities were normalized to control conditions. Data represent the mean ± S.E. (error bars): *, p ≤ 0.05; **, p ≤ 0.005.