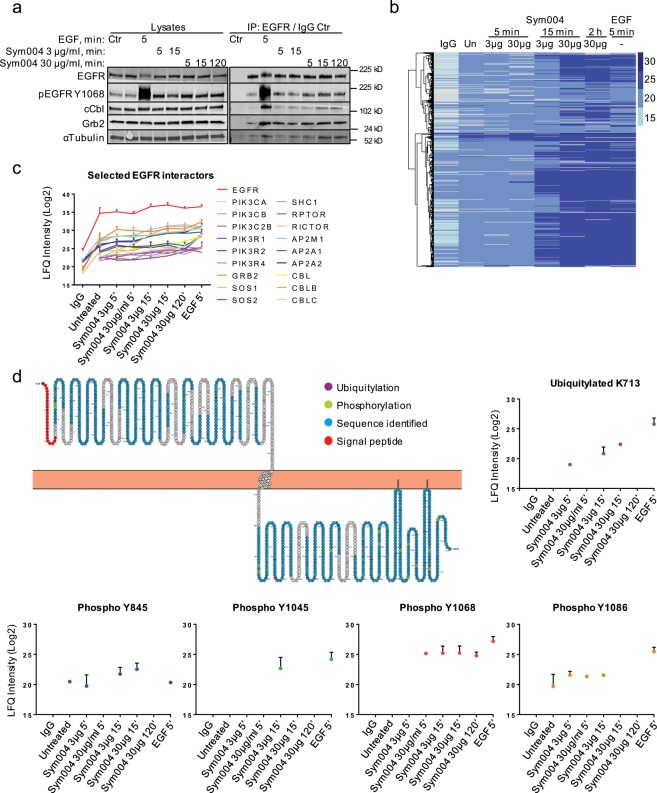
Figure 3.
EGFR interactome upon Sym004 resembles that upon EGF, but differs in kinetics of binding with further differences in EGFR phosphorylation and ubiquitylation events. (a) Serum-starved SCC47 cells were treated with Sym004 (3 µg/ml or 30 µg/ml) for various times (5 min, 15 min or 120 min), or with EGF (50 ng/ml, 5 min), then lysed with mass spectrometric lysis buffer (see Methods). EGFR was immunoprecipitated using EGFR antibody previously cross-linked to beads (normal mouse IgG used in Control). (b) The cells from (a) were subjected to a mass spectrometric sample processing and analysis, and the LFQ protein intensities were subjected to hierarchical clustering analysis to produce a heatmap highlighting differentially abundant proteins in each condition. Darker shades correspond to a relatively high abundance while lighter shades correspond to a relatively low abundance. (c) Examples of selected EGFR interactors. (d) A schematic representation of the EGFR sequence generated using Protter70 showing sequence coverage, all identified phosphorylation and ubiquitylation modifications within EGFR and the signal peptide of 24 amino acids. These 24 amino acids were subsequently subtracted from the identified modifications for the final amino acid numbering. Selected modifications are represented on the graphs. Error bars, SD (if a protein was identified in at least two replicates). LFQ, label-free quantification. All immunoblots were cropped for clarity.