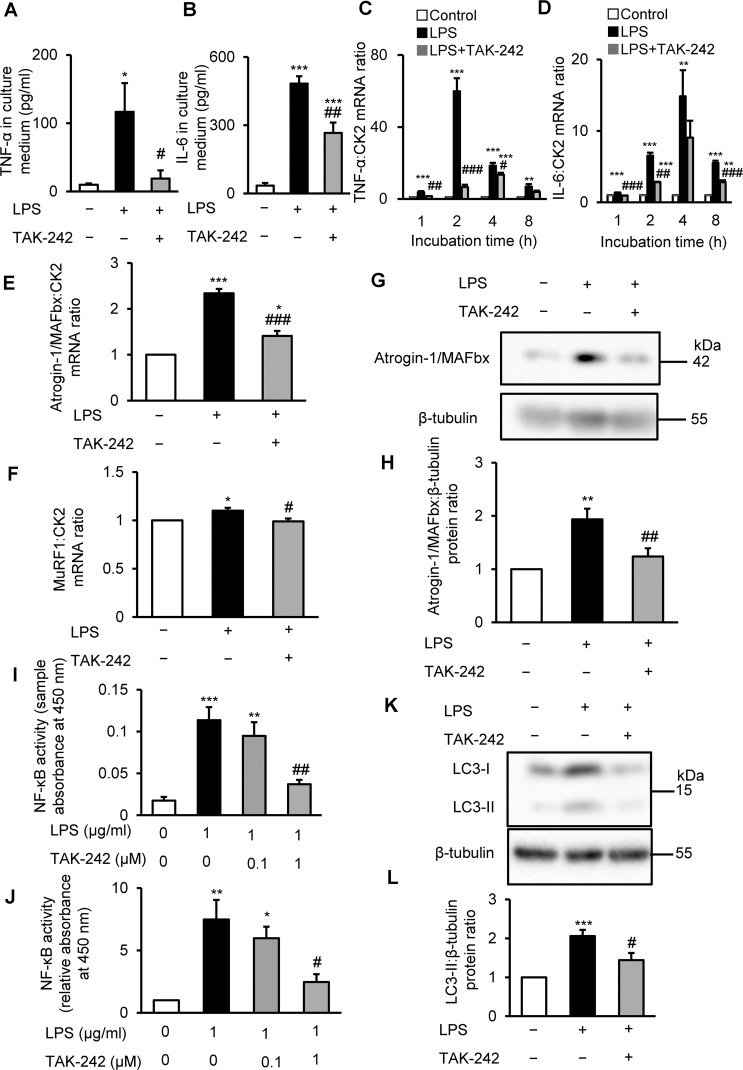
Figure 2.
TAK-242 suppresses LPS-induced activation of inflammatory and proteolytic pathways in C2C12 myotubes. (A,B) C2C12 myotubes were treated with vehicle (0.1% vol/vol DMSO) or TAK-242 (1 μM) and then with PBS or LPS (1 μg/mL) 1 h later. After 4 h, cell culture supernatant was collected and TNF-α (A) and IL-6 (B) concentrations were measured by ELISA. N = 6/group. (C,D) qRT-PCR analysis of TNF-α (C) and IL-6 (D) mRNA levels in C2C12 myotubes treated for up to 8 h as described in (A,B). N = 4/group. (E,F) qRT-PCR analysis of atrogin1/MAFbx (E) and MuRF1 (F) mRNA in C2C12 myotubes treated for 3 h. N = 4–5/group. (C–F) Data were normalised to CK2 mRNA levels and are shown as fold increase over the vehicle-treated controls. (G,H) Western blot analysis (G) and quantification (H) of Atrogin-1/MAFbx in C2C12 myotubes treated for 4 h. Data were normalised to β-tubulin protein levels, and the ratio in vehicle-treated control cells was set at 1.0. N = 7/group. Full-length blots are presented in Supplementary Figure S5A. (I,J) NF-κB (p65) binding activity in C2C12 myotubes treated for 4 h with vehicle, LPS (1 μg/mL), or LPS plus TAK-242 (1 μM and 0.1 μM). NF-κB (p65) DNA-binding activity in myotubes was analysed using a TransAM ELISA kit. Data are shown as sample absorbance at 450 nm (I) or fold increase (J) over the vehicle-treated controls. N = 4/group. (K,L) Western blot analysis (K) and quantification (L) of LC3-II expression in C2C12 myotubes treated for 24 h. Data were normalised to β-tubulin protein levels, and the ratio in vehicle-treated control cells was set at 1.0. N = 14/group. Full-length blots are presented in Supplementary Figure S5B. All panels, data are presented as the mean ± s.e.m. ***p < 0.001, **p < 0.01, *p < 0.05 vs vehicle control; ###p < 0.001, ##p < 0.01, #p < 0.05 vs LPS-treated group. P-values were derived from one-way ANOVA followed by Tukey’s honest significant difference test or Kruskal-Wallis test followed by Dunn’s post hoc tests with Bonferroni correction.