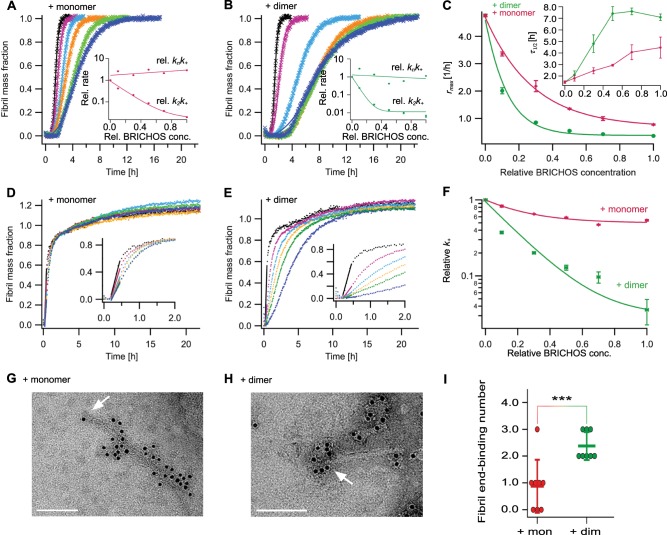
Fig. 3. Effect of rh Bri2 BRICHOS R221E monomers and dimers on the microscopic events of Aβ42 fibril formation.
a, b Individual fits (solid lines) of normalized and averaged aggregation traces (crosses) of 3 µM Aβ42 in the presence of 0 (black), 10 (purple), 30 (cyan), 50 (yellow), 70 (green), and 100% (blue) rh Bri2 BRICHOS R221E monomer a and dimer b with the combined rate constants and as free fitting parameters. The dependencies of the relative combined rate constants obtained from the fits (insets) reveal a strong effect of both rh Bri2 BRICHOS R221E species on secondary nucleation (k+k2) but not on primary (knk+) pathways. c Values for rmax and τ1/2 (inset) extracted from the fitting of Aβ42 aggregation traces in the presence of different concentrations of rh Bri2 BRICHOS R221E species as shown in a and b. d–f Seeded aggregation traces of 3 µM Aβ42 with 0.6 µM preformed Aβ42 fibrils in the presence of 0 (black), 10 (purple), 30 (cyan), 50 (yellow), 70 (green), and 100% (blue) rh Bri2 BRICHOS R221E monomer d and dimer e. f Elongation rates (k+) determined from highly seeded aggregation kinetics in d and e. g–i 5 µM Aβ42 was incubated with and without 50% molar ratio of rh Bri2 BRICHOS R221E monomers g or dimers h overnight at 37 °C. The samples were treated with goat anti-Bri2 BRICHOS antibody and a gold-labeled secondary antibody, and characterized by TEM. The scale bars are 100 nm. The arrows indicate the fibril ends. i Number of gold particles located within 30 nm of eight Aβ42 fibril ends after cofibrillation with rh Bri2 BRICHOS R221E monomers and dimers, respectively. The data are reported as means ± standard deviation. ***p < 0.001.