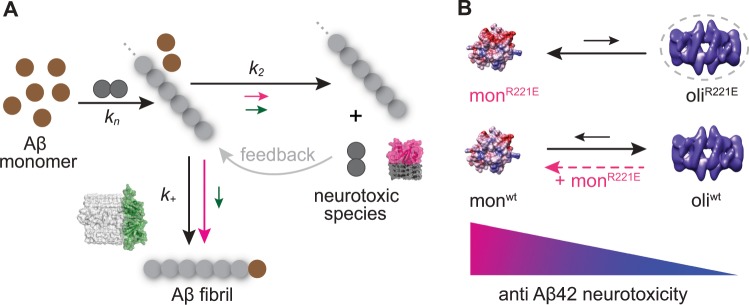
Fig. 5. Model for potentiation of chaperone activity against Aβ42 neurotoxicity by shifting the Bri2 BRICHOS assembly state.
a Aβ42 forms fibrils via primary nucleation, elongation, and secondary nucleation, with rate constants kn, k+, and k2, respectively17. While the Bri2 BRICHOS R221E dimer (green molecular model and green arrows) attenuates both k+ and k2, the monomer (red molecular model and red arrows) predominantly reduces k2. Secondary nucleation catalyses the formation of new nucleation units, which acts as a positive feedback loop (grey arrow) for fibril formation, and this mechanism may be linked to enhanced generation of neurotoxic Aβ42 species18,19. Furthermore, the molecular size of the Bri2 BRICHOS monomer fits well to a single layer of β-structured Aβ42 molecules, which might be the structural element of neurotoxic Aβ42 species. On the contrary, the size of the dimer matches well the area of the cross section of fibril ends (PDB accession code 5KK3), potentially promoting attenuation of the fibril-end elongation rate. The structural properties together with the specific reduction of k2 may thus make the Bri2 BRICHOS monomer most efficient in prevention of Aβ42-associated neurotoxicity. b Rh Bri2 BRICHOS R221E predominately forms monomers and smaller amounts of oligomers (hypothetical model in dashed circle), while rh wild-type (wt) Bri2 BRICHOS mainly assembles into high-molecular weight oligomers (electron microscopy data bank accession code EMD-3918) in equilibrium with monomers. Incubation of rh wt Bri2 BRICHOS oligomers with rh Bri2 BRICHOS R221E monomers destabilizes the oligomers and shifts the kinetic equilibrium toward the monomeric state, leading to an overall increased potency in preventing Aβ42 neurotoxicity (Fig. 2). This model provides thus a basis for understanding how the single point mutation R221E modulates the assembly state of Bri2 BRICHOS and thereby modulates its effects on Aβ42 fibril formation and enhances activities against Aβ42-associated neurotoxicity.