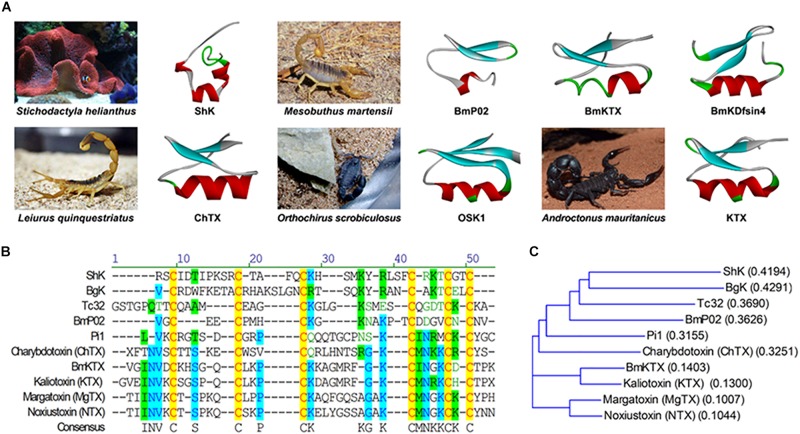
FIGURE 1.
The structures of Kv1.3 blocking toxin peptides. (A) The sea anemone toxin ShK (PDB: 1ROO) is isolated and purified from Sidirodromi hellinikou. The scorpion toxins BmP02 (PDB: 1DU9), BmKTX (PDB: 1BKT), and scorpion defensin BmKDfsin4 (using micasin as a template, PDB: 2LR5) are isolated and purified from Mesobuthus martensii. The scorpion toxin ChTX (PDB: 2CRD) is isolated and purified from Leiurus quinquestriatus. The scorpion toxin OSK1 (PDB: 1SCO) is isolated and purified from Orthochirus scrobiculosus. The scorpion toxin KTX (PDB: 2KTX) is isolated and purified from Androctonus mauritanicus. The three-dimensional structure data of the toxin polypeptide in the figure refers to the PDB. (B) Multiple sequence alignment of ShK and ShK-like Kv1.3 blockers from sea anemone or scorpion venom. Conserved cysteines formatting intrachain disulfide bonds are in red and shadowed in yellow; residues conserved in most of the peptides are shadowed in blue; residues with same charge in most of the peptides are shadowed in green. The species of toxins acting on Kv1.3 are mentioned above, except for Tc32 isolated from Tityus cambridgei; Pi1 isolated from Pandinus imperator; Kaliotoxin isolated from Androctonus mauritanicus; margatoxin isolated from Centruroides margaritatus; noxiustoxin isolated from Centruroides noxius. (C) A guide tree is constructed by ALIGNX, a component of the VECTOR NTI 11.0 software suite. Scores in the brackets are based on the identity of the amino acids chemical properties.