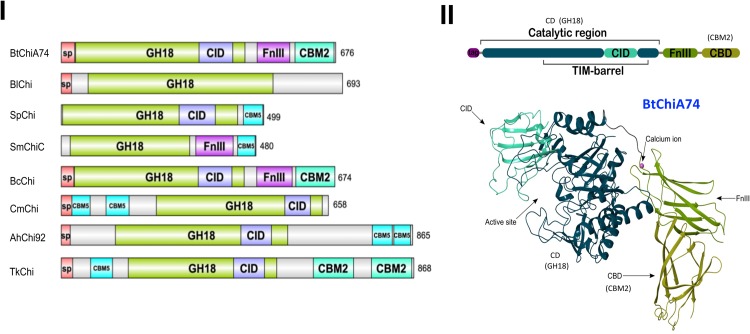
FIGURE 2.
Comparison of the modular structure of chitinases of B. thuringiensis with different bacterial chitinases and the three-dimensional structure of the ChiA74 from B. thuringiensis. (I) Modular alignment between different bacterial chitinases. Protein sequences were analyzed using the Interpro webserver (www.ebi.ac.uk/interpro/beta/) and the figure was built with the program DOG 1.0 (Ren et al., 2009). The nomenclature used in the CAZy database was maintained. Signal peptide, sp; catalytic domains, GH18; CID, chitinase insertion domain; carbohydrate-binding module, CBM; fibronectin type III domain, FnIII. Chitinase (Chi) of: Bt, B. thuringiensis (accession number AF424979.1); Bl, B. licheniformis (QAS14701.1); Sp, Serratia proteamaculans (AGF70636.1); Sm, S. marcescens (ABI79318.1); Sm, S. marcescens AHH32576.1; Bc, B. cereus (FRI-35 AFQ09088.1); Cm, Chitinolyticbacter meiyuanensis (ATN39892.1); Ah, Aeromonas hydrophila (AAG09437.1); Tk, Thermococcus kodakarensis (BAA88380). (II) Crystal structure of the chitinase ChiA74 of B. thuringiensis with little additions in the nomenclature to match with (I). The catalytic region (CD) corresponds to the GH18, and chitin binding domain (CBD) to the CBM2 showed in panel (I). This clarification is also shown in (II). The three-dimensional structure was first reported by our group in Juárez-Hernández et al. (2019), Scientific Reports, available online at https://doi.org/10.1038/s41598-019-39464-z.