Abstract
In the current paper, we aimed to investigate circulating tumor cells (CTCs) in non-small cell lung carcinoma (NSCLC) candidates to immunotherapy and correlate findings with clinical and metabolic parameters. Seventeen metastatic NSCLC patients (12 males, 5 females), were prospectively enrolled. All patients underwent 18F-Fluorodeoxyglucose (FDG) PET/CT and CTCs detection before treatment. CTCs isolation by size was carried out with the ISET method. CTCs were characterized based on cytopathological features and were compared with smoking status, histological subtype, pre-immunotherapy treatment, PDL-1 expression, performance status, and semi-quantitative parameters on PET, including SUVmax, SUVmean, metabolic tumor volume (MTV) and total lesion glycolysis (TLG). We found CTCs in 10 out of 17 patients (59%). Mean number of CTCs was 3 (range 1-7). Only one cell with 3 malignant features was detected in the blood of a healthy control out of 7 (16%). A significantly lower number of CTCs was found in patients previously treated with chemotherapy (P=0.041). No correlation between CTCs and other clinical pathologic characteristics was observed. Patients with an extensive tumor burden, i.e. MTV and TLG, were associated with a higher number of CTCs (P=0.004 and P=0.028, respectively). Likewise, patients with a higher metabolism determined with SUVmean resulted having a higher CTCs count (P=0.048). The presence of CTCs was associated with tumor uptake and metabolic burden on PET/CT, while results were influenced by previous chemotherapy. Whether confirmed in larger series, the combination of the presence of CTCs and FDG PET metabolic parameters might improve prognostic stratification and allow more personalized treatment paradigm.
Keywords: Non-small-cell lung cancer, circulating tumor cells, PET/CT, FDG, immunotherapy, chemotherapy
Introduction
Lung cancer is the leading cause of death worldwide, with non-small-cell lung cancer (NSCLC) representing 80-85% of all cases [1,2]. Due to lacking symptoms at early onset, almost half of the cases are diagnosed in advanced stage [3]. Surgery, chemotherapy, or radiation therapy have been widely used to treat different sub-types of lung cancer. However, up to 50% of patients, even after curative treatment, show tumor recurrence [4-8], suggesting the need for more sensitive diagnostic strategies and biomarkers able to provide prognostic information. Following the clinically relevant results obtained in the last years with immunotherapy in NSCLC patients, checkpoint inhibitors targeting the programmed death-1 (PD-1) and its ligands (PD-Ls) are gradually replacing or combining to “conventional” chemotherapeutic agents [9-13]. Despite the improvement in survival, immunotherapy is not efficacious in all cases and clinicians are still in need of reliable biomarkers for patient selection and response assessment in this setting.
Positron emission tomography/computed tomography with 18F-fluorodeoxyglucose (18F-FDG PET/CT) represents a consolidated and extensively used image modality in the diagnostic work-up of patients with NSCLC [14-16]. At baseline, before any treatment, it provides important information on disease extent and patient prognosis. Currently, this modality is being investigated also in NSCLC patients during the course of immunotherapy [17,18].
In the last years, detection of circulating tumor cells (CTCs) in the bloodstream has emerged as a new potential biomarker, able to monitor treatment efficacy in cancer patients, including NSCLC [19-36]. Krebs and colleagues [24], for example, have shown that stage III and IV NSCLC patients with more than 5 CTCs in 7.5 mL of blood have a worst overall survival (OS) and progression-free survival (PFS). With this regards, tumor metabolic parameters obtained from 18F-FDG PET/CT could be able to predict the presence of CTCs, as previously reported in lung cancer [37-41]. These preliminary data suggest the use of CTCs count also for response assessment to immunotherapy with checkpoint inhibitors. Nevertheless, CTCs detection presents some limits. One of the main limits relates to methodological aspects and concerns sensitivity, specificity, and reproducibility of the data [36]. Moreover, CTC count might be influenced by the clinical history of cancer patients and other tumor-related factors.
Following these premises, in the present study we decided to investigate CTCs count in patients affected by metastatic NSCLC candidate to immunotherapy and assess the relationship between these findings and other clinical and metabolic parameters.
Materials and methods
Patients and study design
The current study has been conducted following the approval of the local IRB and the trial has been registered at https://clinicaltrials.gov/ (First posted: 20/06/2018; NCT03563482).
Between March and November 2017, a total of 17 patients (12 males, 5 females) affected by metastatic or relapsed NSCLC and referred to our Institution for immunotherapy with checkpoint inhibitors (nivolumab and pembrolizumab) were prospectively enrolled. In 6 cases (35%), patients were metastatic at presentation, whereas in the other cases indication to immunotherapy was given after first-line treatment failure. Patients underwent 18F-FDG PET/CT before treatment and CTCs detection from peripheral blood sampling at baseline. As negative control, the blood drawn from 7 healthy donors (3 male, 4 female) was collected. Written informed consent was obtained in all cases. Table 1 summarizes principal characteristics of the patients population.
Table 1.
Principal characteristics of the study cohort
| Nr. | (%) | ||
|---|---|---|---|
| Overall | 17 | 100 | |
| Age | Mean | 72 | |
| Range | 51-87 | ||
| Sex | Female | 5 | 35.7 |
| Male | 12 | 85.7 | |
| Tobacco exposure | Smoker | 3 | 17.6 |
| No smoker | 3 | 17.6 | |
| Former | 10 | 58.9 | |
| NA | 1 | 5.9 | |
| Histology | ADC | 12 | 85.7 |
| SQC | 4 | 28.6 | |
| Other | 1 | 7.1 | |
| Performance status | PS 0 | 9 | 53 |
| PS 1 | 5 | 29.4 | |
| PS 2 | 2 | 11.8 | |
| NA | 1 | 5.8 | |
| Treatment before immunotherapy | CHT | 6 | 64.7 |
| CHT&RT | 5 | 29.4 | |
| SUVmax | Mean | 14 | |
| Range | 5-21 | ||
| SUVmean | Mean | 7 | |
| Range | 3-10 | ||
| TLG | Mean | 868 | |
| Range | 32-3459 | ||
| MTV | Mean | 114 | |
| Range | 9-336 | ||
| Diameter max (mm) | Mean | 55 | |
| Range | 22.2-78.7 | ||
| PD-L1 ≥50 | 5 | 29.4 | |
| CTCs | Mean | 3 | |
| <3 | 12 | ||
| ≥3 | 5 | ||
| Range | 1-7 |
Notes: NC = not classified; NA = not available.
Imaging protocol
PET scans were acquired approximately 60 min after FDG administration in fasting patients, using an activity ranging from 250 to 500 MBq. Whole body images were obtained from the base of the skull to mid-thigh by means of an integrated PET/CT tomograph: GE Discovery PET/CT 690, with an integrated 64-slice CT. Reconstructed images were then displayed on a GE ADW4.6 workstation (GE Healthcare, Waukesha, WI, USA) and interpreted by experienced nuclear medicine physicians. The scanner used in this study is accredited by the EANM Research Ltd (EARL) program, and image analysis was performed using standardized acquisitions [42]. Tumor masses were identified as areas of increased FDG uptake in relation to normal lung parenchyma or other mediastinal structures. Tumor burden was calculated with three-dimensional volumes of interest (VOIs) drawn on the volume of metabolic tumor-related activity by applying a percentage threshold of 42%: maximal standardized uptake value (SUVmax) was defined as the highest pixel value and SUVmean was defined as mean SUV related to the tumor burden. Volumetric parameters included metabolic tumor volume (MTV), estimated from the isoactivity contours drawn automatically on the GE PETVCAR® (PET Volume Computer Assisted Reading), based on a defined threshold, and total lesion glycolysis (TLG), calculated as the product of SUVmean × MTV. All patients investigated in our cohort had more than one lesion. All lesions have been separately analyzed and quantitative data derived from the sum of all tumor volumes. The highest SUVmax of the hottest lesion was considered as SUVmax for the analysis.
CTC isolation and counting
For CTCs detection, 10 ml of blood were collected in EDTA tubes and processed within 2 hours on the Isolation by Size of Tumor/Trophoblastic Cells (ISET) platform (Rarecells, Paris, France). Peripheral blood was filtered through the ISET polycarbonate membrane containing 10 filter-spots with calibrated 8-μm-diameter cylindrical pores, each spot representing the filtration of 1 mL of blood. The membrane was cut into two parts containing 4 and 6 spots per part. Four spots were stained using a freshly made May-Grünwald-Giemsa (MGG) solution according to the technique described by Hofman and colleagues [21] for 5 minutes with undiluted May-Grünwald and subsequently for 5 minutes with 50% diluted May-Grünwald and 40 minutes in 10% diluted Giemsa, followed by rinsing with water. Membranes were then air-dried and mounted with limonene mounting medium (Sigma) and kept in the dark at room temperature. Stained spots were examined under a light microscopy (Olympus BX51) at 10× and subsequently digitized at 40× magnification (Figure 1). All images were analyzed by two cytopathologists blinded to the study data. CTCs were recognized based on four cytopathological features: a) nuclear hyperchromatism, b) increased nuclear volume, c) irregular nuclear borders, and d) increased nucleic ratio/cytoplasm. Cells were defined as CTCs when at least 3 of the above four criteria were fulfilled (Figures 1, 2), as previously described by Hofman and colleagues [21].
Figure 1.
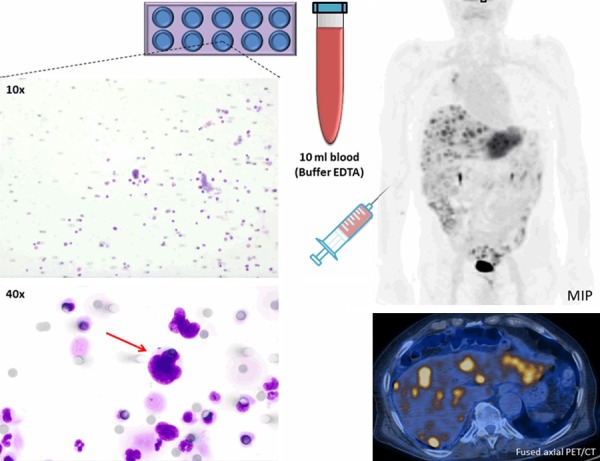
Representative CTC isolated with ISET technique from 10 ml peripheral blood (buffered in EDTA) obtained from a relapsed NSCLC patient with multiple liver metastases. The identified CTC is visualized by MGG staining in 40× magnification (red arrow), and conformed to malignant properties: increased nucleus/cytoplasm ratio, nucleus larger than 3 calibrated pore size of the membrane (>24 μm), irregular nuclear borders, and nuclear hyperchromatism. Round grey spots are 8 μm membrane pores. CTC = circulating tumor cell; MGG = May-Grünwald-Giemsa; ISET = Isolation by Size of Tumor cells; EDTA = Ethylenediaminetetraacetic Acid; MIP = maximal intensity projection.
Figure 2.
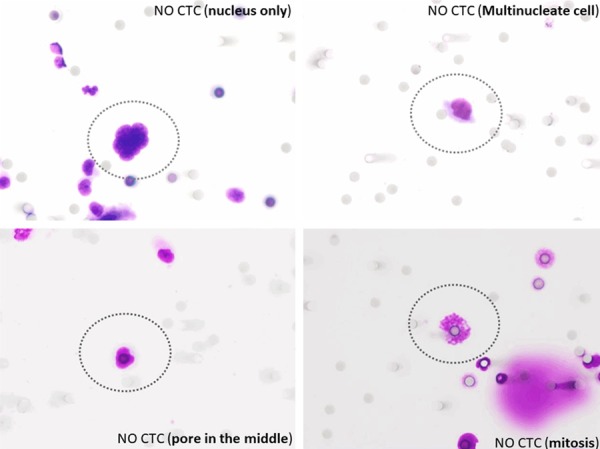
Series of possible findings visualized by MGG staining in 40× magnification that can be confused with CTC and mislead interpretation.
Comparison with other CTCs isolation techniques
PubMed research for circulating tumor cells and lung cancer was performed. Out of the 2778 records on PubMed corresponding to (“neoplastic cells, circulating” [MeSH Terms] OR (“neoplastic” [All Fields] AND “cells” [All Fields] AND “circulating” [All Fields]) OR “circulating neoplastic cells” [All Fields] OR (“circulating” [All Fields] AND “tumor” [All Fields] AND “cells” [All Fields]) OR “circulating tumor cells” [All Fields]) AND (“lung neoplasms” [MeSH Terms] OR (“lung” [All Fields] AND “neoplasms” [All Fields]) OR “lung neoplasms” [All Fields] OR (“lung” [All Fields] AND “cancer” [All Fields]) OR “lung cancer” [All Fields]), we selected clinical studies for (“carcinoma, non-small-cell lung” [MeSH Terms] OR (“carcinoma” [All Fields] AND “non-small-cell” [All Fields] AND “lung” [All Fields]) OR “non-small-cell lung carcinoma” [All Fields] OR “nsclc” [All Fields]) investigated with the similar methodology based on isolation by size, i.e. ISET (Rarecells, Paris, France) and ScreenCell (Sarcelles, France).
Statistical analysis
The total CTCs count, along with mean and median values was calculated. The presence or absence of CTCs was compared with the patients’ baseline epidemiological and clinical-pathologic characteristics, including age, gender, smoking status, histologic subtype, chemotherapy pre-immunotherapy, PDL-1 status, performance status, as well as with metabolic indexes on 18F-FDG PET/CT, comprising SUVmax, SUVmean, MTV and TLG. Associations of CTCs with clinical and metabolic characteristics were studied using Fisher’s exact or Student T-test, when appropriate. Chi-square test was applied to score significant difference between CTCs groups. The ANOVA test was used to explore the association between the CTCs number, analyzed as a continuous variable, and clinical parameters. Bonferroni correction was applied to verify significance for multiple testing. All data were indicated as mean ± SD. All statistical analyses were performed with GraphPad Prism (version 7).
Results
CTCs counting and clinical parameters
CTCs were found in 10 out of 17 patients (59%). The mean ± SD number of CTCs was 3±2, 4/4 mL, while median was 2/4 mL, (range 1-7 CTCs/4 ml) in NSCLC patients. Only one cell with 3/4 malignant features was detected in the blood of a healthy volunteer out of 7 donors analyzed (16%). Based on previous research by Hofman [21], patients were categorized as follows according to CTCs number: Group 1, less than 3 CTCs in 4 spots (equivalent to 4 ml of blood sample) and Group 2, 3 or more than 3 CTCs in 4 spots. Before immunotherapy, 11 (64.7%) patients have been treated with chemotherapy only or chemotherapy plus radiation therapy (Table 1). A significant association was observed between CTCs and prior chemotherapy status. In particular, patients who had undergone chemotherapy were characterized by a lower number of CTCs than those who had not performed chemotherapy (P=0.041, Figure 3). No significant difference in terms of cytopathologic characteristics of CTCs between adenocarcinoma and other lung histotypes was also found. Additionally, we did not find any correlation between CTCs and other epidemiologic and clinical-pathologic parameters (age, gender, tobacco history, tumor size, PD-L1 status). Likewise, no significant difference was found between CTCs number and organs interested by metastases.
Figure 3.

Representative plots comparing the number of CTCs to other clinical and metabolic parameters; Mean number of CTCs resulted significantly associated with increased TLG and MTV (P=0.028, P=0.004 respectively) and concordant with higher SUVmean (P=0.048). The presence of CTCs was significantly reduced in patients who had previously undergone chemotherapy (P=0.041).
CTCs counting and PET-derived parameters
A statistically significant association with the presence of CTCs was found for semi-quantitative and tumor burden parameters on PET/CT (Figure 4). Patients with an extended tumor burden, expressed by TLG and MTV, were associated with higher number of CTCs. We found a mean TLG of 649.8±187.5 in patients with low CTCs counting (<3) compared to 2510±603.7 in those with a number of CTCs ≥3 (P=0.028, Figure 3). Additionally, when compared to patients with low CTCs (<3), patients with ≥3 CTCs had higher MTV values (3541±1560 cm3 vs 19134±9749 cm3, respectively P=0.004). Likewise, SUVmean was statistically higher in patients with number of CTCs ≥3 compared to those with less CTCs (8.8±7.9 vs 5.5±8.4, P=0.048) (Figure 3). No significant difference of SUVmax was observed in both Groups. After correction for multiple testing, CTCs count was confirmed as significantly correlated to MTV values.
Figure 4.
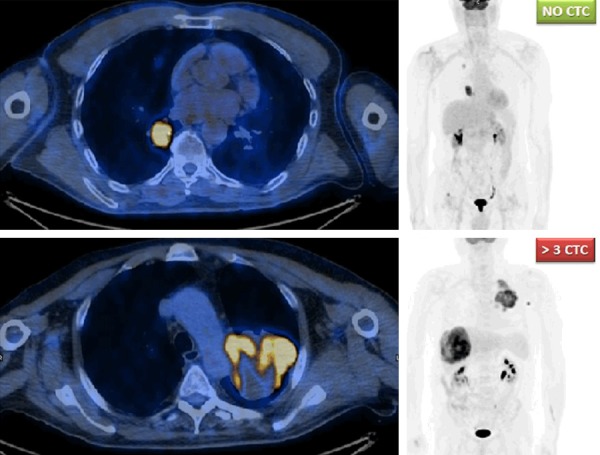
Comparison of two cases with diverse CTCs count and tumor burden at baseline: MIP (maximal intensity projection) images shown on the right side; axial fused PET/CT images at the level of lung lesions are shown on the left. The upper case had no evidence of CTCs at peripheral blood stream, while the lower case had more than 3 CTCs (more precisely, 4 CTCs counted).
Correlation of metabolism with other parameters
The distribution of PET variables in the study cohort is shown in Table 1. We observed a higher MTV and TLG in NSCLC patients with positive PD-L1 staining at immunohistochemistry (P=0.002 and 0.003, respectively). No significant difference was observed for SUVmax and SUVmean and tumor PD-L1 expression. Patient who did not performed chemotherapy had in general a significantly higher MTV and TLG compared to those previously treated (P=0.034 and 0.007, respectively), although there we no statistically significant difference in terms of SUVmax (P=0.574) and SUVmean (P=0.117). Also correlation to other clinical-pathological parameters showed no statistically significant difference with respect to PET-derived variables.
PubMed research results
Overall, 23 papers fulfilled the research criteria. ISET (Rarecells, Paris, France) technology was used in 14 cases, out of which 6 were performed in comparison to CellSearch (EpCam-based). ScreenCell (Sarcelles, France) technology was used in 9 cases. The records obtained from PubMed research and study results are illustrated in Table 2.
Table 2.
Summary of the available articles analyzing CTCs by morphology in patients with non-small cell lung cancer
| Author | Tumor | Nr. of cancer patient | Nr. of patients with CTCs | CTCs/ml mean | CTCs/ml range | Nr. of healthy donors | Nr. of health donors with CTCs | CTC isolation technique | Findings |
|---|---|---|---|---|---|---|---|---|---|
| Farace F et al. 2011 [43] | Lung Cancer | 20 | 20/20 (100%) by ISET; 9/20 (45%) by CellSearch | 1.6 by ISET; 90.4 by CellSearch | 0.1-13 by ISET; 0-1800 by CellSearch | n.a. | n.a. | CellSearch and ISET | Concordant results between ISET and CellSearch in only 20% of patients. |
| Hofman V et al. 2011 [21] | Resectable NSCLC | 208 | 76 (36%) | 7 | 0-83 | 39 | 0 (0%) | ISET | The number of CNHCs is significantly associated with shorter OS and DFS. |
| Hofman V et al. 2011 [22] | Resectable NSCLC | 210 | 82 (39%) by CellSearch, 104 (50%) by ISET | 1.7 by CellSearch; 4.9 by ISET | 1-3, 3 | 40 | 0 (0%) by CellSearch and ISET | CellSearch and ISET | The number of CNHCs is significantly associated with shorter DFS. |
| Hofman V et al. 2011 [23] | NSCLC (among many benign and malignant disease) | 394 | 119 (30%) | n.a. | n.a. | 49 | 0 (0%) | ISET | CNHCs detected on filters were usually higher in patients with malignant diseases than the number observed in patients with nonmalignant diseases (P<.001). |
| Lecharpentier A. et al. 2011 [43] | NSCLC | 6 | 6 (100%) | 8.7 | 1.6-19 | n.a. | n.a. | ISET | Vimentin and keratin double positive CTCs, corrisponding to hybrid epithelial/mesenchymal phenotype, were found in all patients. |
| Hofman V et al. 2012 [27] | Resectable NSCLC | 250 | 102 (41%) | na | na | 59 | 0 (0%) | ISET | Interobserver variation among 10 pathologists was low for the diagnosis of malignant cells. |
| Krebs M G et al. 2012 [25] | Stage III and IV NSCLC | 40 | 9 (23%) by CellSearch; 32 (80%) by ISET | 0.05±0.5 by CellSearch; 2±8 by ISET | 0-139 | n.a. | n.a. | CellSearch and ISET | There was no statistical concordance between the numbers of CTCs detected by the two techniques. |
| Pailler E et al. 2013 [34] | ALK-positive and negative NSCLC | 18 ALK+ and 14 ALK- | 18/18 (100%) ALK+ CTCs | 9 | 4-46 by ISET; 0-11 by CellSearch | n.a. | n.a. | ISET and CellSearch | ALK rearrangment can be detected in CTCs of patients ALK-positive. |
| Ilie M et al. 2014 [26] | 168 COPD | / | 5/168 (3%) COPD | 9.4 | 3-11 | 77 | 0 (0%) | ISET | The presence of CTC was significantly correlated to the severity of COPD (P<0.001). |
| High risk individuals | |||||||||
| Freidin MB et al. 2014 [32] | Primary lung cancer patients undergoing surgery (32) + pulmonary metastasis (21) | 32 + 21 | 56-65% in primary LC; 47-71% in metastatic patient | n.a. | n.a. | 17 Benign Lung lesion | 35-29% | Scree Cell | Lung cancer patients have more CTCs that benign lesions. |
| Pailler E et al. 2015 [45] | ROS1 rearranged and ROS1-negative NSCLC | 8 | 8/8 (100%) by ISET; 4/8 (50%) by CellSearch | 8.4 (ISET-ROS1 rearranged); 0.2 (CellSearch) | 2.3-18.3 (ISET); 0-1 (CellSearch) | n.a. | n.a. | CellSearch and ISET | ROS1 rearrangement can be detect in CTCs and can predict resistance to ROS1-ihibitor therapy. |
| Chudasama D et al. 2015 [46] | Lung Cancer before; and after radiofrequency ablation | 9 | 3/9 (33%) before; 7/9 (78%) after cryotherapy | 1 before; >10 after cryotherapy | 0-17 before; 0->33 after cryotherapy | n.a. | n.a. | ScreenCell | Increased CTC count after radiofrequency ablation (larger increase in metastatic patients). |
| Chudasama D et al. 2015 [47] | Lung Cancer before; and after cryotherapy | 20 | 5/20 (25%) before; 15/20 (75%) after cryotherapy | 1 before; >10 after cryotherapy | 0-17 before; 0->33 after cryotherapy | n.a. | n.a. | ScreenCel | Increased CTC count after cryotherapy. |
| Fiorelli A et al. 2015 [48] | Lung Cancer | 60 | 54 (90%) | 17.3±11.6 | 17.3±11.6 | 17 | 1 (6%) | ScreenCell | Moderate correlation between SUV value and CTC count. |
| The presence of >3.3 CTCs/ml is associated with malignant lesion. | |||||||||
| Mascalchi M et al. 2016 [33] | Stage III-IV lung cancer before FNA biopsy | 28 | 17/28 (65%) | 2.26±1.2 | 0-15/mL | n.a. | n.a. | ScreeCell | No correlation between number of CTC or CTM and tumor type or stage was observed. |
| Sawabata N et al. 2016 [49] | Pre and post-operative NSCLC | 23 | 7/23 (30%) before surgery | 7/23 (30%) before surgery | 1.6 before surgery | 0-8 before surgery | n.a. | Screen Cell | CTCs detection may increase during surgical manipulation and authors found a correlation with clinical parameters (such as SUVmax). |
| 17/23 (74%) after surgery | 17/23 (74%) after surgery 1/23 (4%) 6 h after surgery | 1.6 after surgery | 0-3 after surgery | ||||||
| 1/23 (4%) 6 h after surgery | 19/23 (83%) pulmonary vein blood collection | 0.04 6 h after surgery | 0-0.3 6 h after surgery | ||||||
| 19/23 (83%) pulmonary vein blood collection | 3.13 pulmonary vein blood collection | 0-4 pulmonary vein blood collection | |||||||
| Coco S et al. 2017 [28] | Stage IIIB and IV NSCLC | 73 | 34>2 CTC/ml (39 hanno ≤2 CTC/mL) | 2 | 0-16.6 | n.a. | n.a. | Screen Cell Cyto | The presence of CTC was associated to better OS (opposite to expectation). |
| Chudasama D et al. 2017 [31] | NSCLC undegoing surgical tratment. 13 adeno, 10 squamous; 18 early stage, 5 stage III-IV | 23 | 80.6% of early stage, 60% of late stage | n.a. | n.a. | n.a. | n.a. | Screen Cell | The presence of CTC correlates to better OS (P<0.0009; opposite to expectation). |
| Ilie M et al. 2017 [50] | Stage III-IV NSCLC | 256 by CellSearch; 106 by ISET | 113/256 (44%) and 80/106 (75%) | 15 by ISET (among CTC+) | 0-35 by CellSearch; 0-64 by ISET | n.a. | n.a. | CellSearch and ISET | ISET approach identifies a higher proportion of CTC+ patient than CellSearch. MET status in ISET-captured CTCs correlate with MET status in tumor tissue. |
| Mascalchi M et al. 2017 [51] | Lung Cancer | 67 | 47/67 (70%) | 0.7 | 0-4 | 8 | 1/8 (12%) | Screen Cell | Due to low sensitivity, the search of CTCs cannot replace CT guided percutaneous FNA or core biopsy. |
| Kallergi G et al. 2018 [29] | Chemo-naive stage IV NSCLC | 30 | 93.3% (28/30). After chemotherapy 81.8% (9/11) | 5 | 0-23 CTCs/ml | n.a. | n.a. | ISET | Significant correlation between CK-positive (IF) and Giemsa-positive tumor cells (P=0.001). |
| Ilié M et al. 2018 [35] | NSCLC | 106 | 80 (75%) | 15 | 0.5-64 | n.a. | n.a. | ISET | 93% concordance between PD-L1 status in tissue and CTCs. A trend for longer PFS was observed in cases with PD-L1 expression in CTCs or WBCs (P=0.2). |
| Ilié M et al. 2018 [52] | Lung Adenocarcinoma | 36 (validation set) | 27/36 (75%, EDTA collection) | 1.25 (EDTA) | 0.25-19 (EDTA) | 10 | n.a. | ISET | BCT blood collection tubes preserved morphology of CTCs, when compared to EDTA tubes, and allowed ICC for MET and FISH for ALK rearrangement. |
| 29/36 (81%, BCT collection) | 2.75 (BCT) | 0.25-21 (BCT) |
Discussion
In this work we aimed to investigate CTCs count in NSCLC cancer patients candidate to immunotherapy in order to first assess whether there is an association with metabolic and other clinical parameters. In the last years, many studies have described the detection of CTCs in lung cancer patients with different approaches and objectives (e.g. diagnostic or prognostic). We focused our attention on filter-based isolation techniques, i.e. ISET (Rarecells, Paris, France) and ScreenCell (Sarcelles, France) that in comparative studies have shown an overall higher sensitivity than marker-based approaches, i.e. CellSerach (EpCam-based) [22,25,34,43,50]. In fact, ISET is capable to identify CTCs in 76% (range: 50-100%) of lung cancer patients, whereas CellSearch can identify CTCs in 36% of the cases (on average, range; 23-45%) [22,25,43,50]: The lack of statistical concordance between the CTCs count detected by the two techniques [25] suggests that they might identify different cancer cell subpopulations.
CTCs isolated by size have been extensively characterized for the expression of common epithelial/mesenchymal markers, such as Vimentin and keratin [29,44], but also for lung cancer specific biomarkers, such as ALK rearrangement [34,52], PD-L1, ROS1 and MET expression [35,50,52]. In particular, Iliè and colleagues have found a 93% concordance between PD-L1 status in tissue and CTCs and a trend for longer PFS was observed in cases with PD-L1 expression in CTCs or WBCs (P=0.2) [35].
These studies raised the possibility to use CTCs as a monitor of treatment efficacy in NSCLC patients: CTCs count has been investigated during surgical manipulation [49], during stereotactic body radiation therapy (SBRT) [53], after radiofrequency ablation [46] and after cryotherapy [47]. CTCs have emerged as an important tumor biomarker for a wide range of human cancers [54-59] and might find a proper place in treatment regimens based on checkpoint inhibitors.
In this preliminary study, we evaluated at first the relationship between the number of CTCs and a series of epidemiological and clinical characteristics in a cohort of patients with metastatic or relapsed NSCLC candidate to immunotherapy. In our cohort, CTCs were found in 59% of patients with a mean density of 3/4 mL blood, which is consistent with the results of two previous studies that investigated subjects candidate to chemotherapy as first-line regimen [60,61]. Although the authors used an alternative method for CTC detection, in these same studies, CTCs at baseline and during follow-up resulted a strong independent predictor of survival in advanced NSCLC receiving chemotherapy. No other clinical parameters were associated with the number of CTCs. Also Hofman et al. [21] did not observe any significant correlation between the density of CTCs and disease stage or other clinical parameters (i.e., age, gender, tobacco exposure, tumor size, histologic subtype, and histologic grade). Similar to Krebs et al. [24], we found that the presence and the number of CTCs were influenced by previous cycles of chemotherapy. Indeed, patients with a positive history for previous therapy showed lower levels of CTCs compared to those who had not undergone prior chemotherapeutic treatment (P=0.041). Consequently, CTCs count after chemotherapy in the bloodstream might determine the grade of response and, on the other hand, could be used to characterize any morphological or genetic modification occurring in tumor cells after systemic treatment to determine those at greater risk of disseminated disease.
We also explored potential correlations between the density of CTCs and PET-derived metabolic parameters in NSCLC patients. Our findings revealed a significant relationship between higher densities of CTCs and tumor metabolic activity expressed by high levels of SUVmean. Additionally, the estimated tumor burden, expressed by TLG and MTV, was significantly associated with the number of CTCs (P=0.028 and P=0.048, respectively). These results suggest that the density of CTCs in the peripheral bloodstream can reflect the tumor burden and provide valuable information on the metabolic activity, which may serve as a marker of tumor aggressiveness in metastatic NSCLC. PD-L1 expression on the other side did not correlate to CTCs count, whereas evidence of other therapeutic regimens resulted in our study correlated to both CTCs count and volumetric PET parameters (i.e. MTV and TLG). The fact that FDG uptake correlates to PD-L1 in the tumor [17,62,63] does not necessarily mean that also CTCs number should correlate. Differently, we would expect an association between PD-L1 levels on CTCs to the tumor PD-L1 expression [35]. The two aspects, in fact, could provide independent information on tumor prognosis, particularly with respect to immunotherapy outcome. Previously, the relationship between CTCs and 18F-FDG PET/CT has been assessed in different types of malignancies, including lung cancer [37-41,64-66]. In a cohort of NSCLC chemotherapy naïve patients, Nair et al. [37] and Morbelli et al. [38] have showed that only SUVmax was associated with CTCs, whereas Nygaard et al. [64] demonstrated a worse survival in NSCLC patients with higher MTV and higher levels of plasm cell-free DNA (cfDNA), although no significant correlation between PET parameters and cfDNA was detected. A significant correlation for post-operative CTCs count with SUVmax, pathological stage and surgical approach was demonstrated instead by Bayarri-Lara et al. [67] in 102 stage I-IIIA NSCLC patients. In this cohort, SUVmax resulted the only independent predictor for CTC presence after the operation. Similarly to our findings, Nair et al. [37] have reported no correlation for CTCs and tumor diameter in treatment naïf NSCLC patients. On counterpart, volumetric parameters in our cohort (i.e. MTV and TLG) resulted significantly different in NSCLC patients with more than 3 CTCs compared to those having less. In particular, MTV was confirmed predictive also after correction for multiple testing. In Fiorelli et al. [48], instead, CTCs count resulted significantly correlated to tumor stage and size, and moderately associated to SUV value (Table 2). Herein, the presence of more than 3.3 CTCs/ml (25/7.5 ml) in the bloodstream resulted predictive of malignancy in patients with lung lesions. On the other hand, considering patients as responders vs no-responders by PET/CT after erlotinib and pertuzumab, Punnoose et al. [65] highlighted higher levels of CTCs in patients classified as no-responders, suggesting CTCs as an early, non-invasive predictor of response.
Our report is one of the first to examine the association between CTCs and PET parameters in the era of immune checkpoint inhibitors. The present study has anyhow some limitations: at first, it includes a limited number of patients, due to its preliminary nature, making definite conclusions difficult. Secondarily, the lack of extensive follow-up prevents any hypothesis on the predictive and prognostic role of decreasing CTC-levels, and PET-derived parameters during immunotherapy. Thirdly, we did not collect other circulating markers, such as cfDNA, which are known as potential biomarkers in cancer.
Despite the limitations, our study confirms the expectations on CTCs count in NSCLC patients and its correlation with other factors, such as PET-parameters. Future investigations should focus on the use of all these factors to predict response and outcome in patients under immunotherapy.
Conclusions
The presence of CTCs identified by ISET is influenced by previous chemotherapy and may be a reflection of tumor biology and metabolism in metastatic NSCLC prior to checkpoint inhibitors. Future prospective studies will be needed to confirm whether this non-invasive diagnostic tool is of additional value in driving the best treatment and establishing individual prognostic outcomes. An interesting point might be to evaluate CTCs genetic profile and compare its characteristics with those of primary tumor and other circulating tumor markers.
Acknowledgements
The authors want to thank AIRC Foundation for the support on this research. ISET machine is available thanks to a grant from LILT (Lega Italiana per la Lotta contro i Tumori). This study was funded by Fondazione AIRC (Associazione Italiana per la Ricerca sul Cancro) with the grant number Nr.18,923 provided to E.L.
Disclosure of conflict of interest
E.L. reports receiving an individual grant from AIRC and the Italian Ministry of Health. A.C. is supported by fellowships provided by AIRC. S.M. reports fellowship support from Fondazione Umberto Veronesi. G.V. reports receiving personal fees from Ab Medica SpA, and grants from AIRC, the Ministry of Health, the Italian National Insurance Institute for Workplace Injuries (INAIL), and the National Cancer Institute (NCI).
Abbreviations
- CTCs
circulating tumor cells
- NSCLC
non-small cell lung cancer
- 18F-FDG PET
18-fluorodeoxyglucose positron emission tomography
- CT
computed tomography
- ISET
isolation by size of Tumor/Trophoblastic Cells
- PETVCAR®
PET Volume Computer Assisted Reading
- SUV
standardized uptake value
- MTV
metabolic tumor volume
- TLG
total lesion glycolysis
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Blanchon F, Grivaux M, Asselain B, Lebas FX, Orlando JP, Piquet J, Zureik M. 4-year mortality in patients with non-small-cell lung cancer: development and validation of a prognostic index. Lancet Oncol. 2006;7:829–836. doi: 10.1016/S1470-2045(06)70868-3. [DOI] [PubMed] [Google Scholar]
- 3.Chansky K, Detterbeck FC, Nicholson AG, Rusch VW, Vallières E, Groome P, Kennedy C, Krasnik M, Peake M, Shemanski L, Bolejack V, Crowley JJ, Asamura H, Rami-Porta R IASLC Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions. The IASLC lung cancer staging project: external validation of the revision of the TNM stage groupings in the eighth edition of the TNM classification of lung cancer. J Thorac Oncol. 2017;12:1109–21. doi: 10.1016/j.jtho.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Goya T, Asamura H, Yoshimura H, Kato H, Shimokata K, Tsuchiya R, Sohara Y, Miya T, Miyaoka E Japanese Joint Committee of Lung Cancer Registry. Prognosis of 6644 resected non-small cell lung cancers in Japan: a Japanese lung cancer registry study. Lung Cancer. 2005;50:227–34. doi: 10.1016/j.lungcan.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 5.van Rens MT, de la Rivière AB, Elbers HR, van den Bosch JM. Prognostic assessment of 2,361 patients who underwent pulmonary resection for non-small cell lung cancer, stage I, II, and IIIA. Chest. 2000;117:374–9. doi: 10.1378/chest.117.2.374. [DOI] [PubMed] [Google Scholar]
- 6.Naruke T, Tsuchiya R, Kondo H, Asamura H. Prognosis and survival after resection for bronchogenic carcinoma based on the 1997 TNM-staging classification: the Japanese experience. Ann Thorac Surg. 2001;71:1759–64. doi: 10.1016/s0003-4975(00)02609-6. [DOI] [PubMed] [Google Scholar]
- 7.Pfannschmidt J, Muley T, Bulzebruck H, Hoffmann H, Dienemann H. Prognostic assessment after surgical resection for non-small cell lung cancer: experiences in 2083 patients. Lung Cancer. 2007;55:371–7. doi: 10.1016/j.lungcan.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Yasumoto K, Hanagiri T, Takenoyama M. Lung cancer-associated tumor antigens and the present status of immunotherapy against non-small-cell lung cancer. Gen Thorac Cardiovasc Surg. 2009;57:449–457. doi: 10.1007/s11748-008-0433-6. [DOI] [PubMed] [Google Scholar]
- 9.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 11.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR KEYNOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1-positive nonsmall-cell lung cancer. N Engl J Med. 2016;375:1823–33. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 12.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, Cortinovis DL, Leach J, Polikoff J, Barrios C, Kabbinavar F, Frontera OA, De Marinis F, Turna H, Lee JS, Ballinger M, Kowanetz M, He P, Chen DS, Sandler A, Gandara DR OAK Study Group. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–65. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossi S, Toschi L, Castello A, Grizzi F, Mansi L, Lopci E. Clinical characteristics of patient selection and imaging predictors of outcome in solid tumors treated with checkpoint-inhibitors. Eur J Nucl Med Mol Imaging. 2017;44:2310–2325. doi: 10.1007/s00259-017-3802-5. [DOI] [PubMed] [Google Scholar]
- 14.Liao S, Penney BC, Wroblewski K, Zhang H, Simon CA, Kampalath R, Shih MC, Shimada N, Chen S, Salgia R, Appelbaum DE, Suzuki K, Chen CT, Pu Y. Prognostic value of metabolic tumor burden on 18F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2012;39:27–38. doi: 10.1007/s00259-011-1934-6. [DOI] [PubMed] [Google Scholar]
- 15.Kahraman D, Holstein A, Scheffler M, Zander T, Nogova L, Lammertsma AA, Boellaard R, Neumaier B, Dietlein M, Wolf J, Kobe C. Tumor lesion glycolysis and tumor lesion proliferation for response prediction and prognostic differentiation in patients with advanced non-small cell lung cancer treated with erlotinib. Clin Nucl Med. 2012;37:1058–1064. doi: 10.1097/RLU.0b013e3182639747. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Dong M, Sun X, Li W, Xing L, Yu J. Prognostic value of 18F-FDG PET/CT in surgical non-small cell lung cancer: a meta-analysis. PLoS One. 2016;11:e0146195. doi: 10.1371/journal.pone.0146195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grizzi F, Castello A, Lopci E. Is it time to change our vision of tumor metabolism prior to immunotherapy? Eur J Nucl Med Mol Imaging. 2018;45:1072–1075. doi: 10.1007/s00259-018-3988-1. [DOI] [PubMed] [Google Scholar]
- 18.Kaira K, Higuchi T, Naruse I, Arisaka Y, Tokue A, Altan B, Suda S, Mogi A, Shimizu K, Sunaga N, Hisada T, Kitano S, Obinata H, Yokobori T, Mori K, Nishiyama M, Tsushima Y, Asao T. Metabolic activity by (18)F-FDG-PET/CT is predictive of early response after nivolumab in previously treated NSCLC. Eur J Nucl Med Mol Imaging. 2018;45:56–66. doi: 10.1007/s00259-017-3806-1. [DOI] [PubMed] [Google Scholar]
- 19.Paterlini-Brechot P, Benali NL. Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer Lett. 2007;253:180–204. doi: 10.1016/j.canlet.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Coello MC, Luketich JD, Litle VR, Godfrey TE. Prognostic significance of micrometastasis in non-small-cell lung cancer. Clin Lung Cancer. 2004;5:214–25. doi: 10.3816/CLC.2004.n.002. [DOI] [PubMed] [Google Scholar]
- 21.Hofman V, Bonnetaud C, Ilie MI, Vielh P, Vignaud JM, Fléjou JF, Lantuejoul S, Piaton E, Mourad N, Butori C, Selva E, Poudenx M, Sibon S, Kelhef S, Vénissac N, Jais JP, Mouroux J, Molina TJ, Hofman P. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res. 2011;17:827–35. doi: 10.1158/1078-0432.CCR-10-0445. [DOI] [PubMed] [Google Scholar]
- 22.Hofman V, Ilie MI, Long E, Selva E, Bonnetaud C, Molina T, Vénissac N, Mouroux J, Vielh P, Hofman P. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: comparison of the efficacy of the cellsearch assay™ and the isolation by size of epithelial tumor cell method. Int J Cancer. 2011;129:1651–60. doi: 10.1002/ijc.25819. [DOI] [PubMed] [Google Scholar]
- 23.Hofman VJ, Ilie MI, Bonnetaud C, Selva E, Long E, Molina T, Vignaud JM, Fléjou JF, Lantuejoul S, Piaton E, Butori C, Mourad N, Poudenx M, Bahadoran P, Sibon S, Guevara N, Santini J, Vénissac N, Mouroux J, Vielh P, Hofman PM. Cytopathologic detection of circulating tumor cells using the isolation by size of epithelial tumor cell method: promises and pitfalls. Am J Clin Pathol. 2011;135:146–56. doi: 10.1309/AJCP9X8OZBEIQVVI. [DOI] [PubMed] [Google Scholar]
- 24.Krebs MG, Sloane R, Priest L, Lancashire L, Hou JM, Greystoke A, Ward TH, Ferraldeschi R, Hughes A, Clack G, Ranson M, Dive C, Blackhall FH. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J. Clin. Oncol. 2011;29:1556–63. doi: 10.1200/JCO.2010.28.7045. [DOI] [PubMed] [Google Scholar]
- 25.Krebs MG, Hou JM, Sloane R, Lancashire L, Priest L, Nonaka D, Ward TH, Backen A, Clack G, Hughes A, Ranson M, Blackhall FH, Dive C. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol. 2012;7:306–15. doi: 10.1097/JTO.0b013e31823c5c16. [DOI] [PubMed] [Google Scholar]
- 26.Ilie M, Hofman V, Long-Mira E, Selva E, Vignaud JM, Padovani B, Mouroux J, Marquette CH, Hofman P. “Sentinel” circulating tumor cells allow early diagnosis of lung cancer in patients with chronic obstructive pulmonary disease. PLoS One. 2014;9:e111597. doi: 10.1371/journal.pone.0111597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofman V, Long E, Ilie M, Bonnetaud C, Vignaud JM, Fléjou JF, Lantuejoul S, Piaton E, Mourad N, Butori C, Selva E, Marquette CH, Poudenx M, Sibon S, Kelhef S, Vénissac N, Jais JP, Mouroux J, Molina TJ, Vielh P, Hofman P. Morphological analysis of circulating tumour cells in patients undergoing surgery for non-small cell lung carcinoma using the isolation by size of epithelial tumour cell (ISET) method. Cytopathology. 2012;23:30–8. doi: 10.1111/j.1365-2303.2010.00835.x. [DOI] [PubMed] [Google Scholar]
- 28.Coco S, Alama A, Vanni I, Fontana V, Genova C, Dal Bello MG, Truini A, Rijavec E, Biello F, Sini C, Burrafato G, Maggioni C, Barletta G, Grossi F. Circulating cell-free DNA and circulating tumor cells as prognostic and predictive biomarkers in advanced non-small cell lung cancer patients treated with first-line chemotherapy. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18051035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kallergi G, Vetsika EK, Aggouraki D, Lagoudaki E, Koutsopoulos A, Koinis F, Katsarlinos P, Trypaki M, Messaritakis I, Stournaras C, Georgoulias V, Kotsakis A. Evaluation of PD-L1/PD-1 on circulating tumor cells in patients with advanced non-small cell lung cancer. Ther Adv Med Oncol. 2018;10:1758834017750121. doi: 10.1177/1758834017750121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanssen A, Wagner J, Gorges TM, Taenzer A, Uzunoglu FG, Driemel C, Stoecklein NH, Knoefel WT, Angenendt S, Hauch S, Atanackovic D, Loges S, Riethdorf S, Pantel K, Wikman H. Characterization of different CTC subpopulations in non-small cell lung cancer. Sci Rep. 2016;6:28010. doi: 10.1038/srep28010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chudasama D, Barr J, Beeson J, Beddow E, McGonigle N, Rice A, Nicholson A, Anikin V. Detection of circulating tumour cells and survival of patients with non-small cell lung cancer. Anticancer Res. 2017;37:169–173. doi: 10.21873/anticanres.11302. [DOI] [PubMed] [Google Scholar]
- 32.Freidin MB, Tay A, Freydina DV, Chudasama D, Nicholson AG, Rice A, Anikin V, Lim E. An assessment of diagnostic performance of a filter-based antibody-independent peripheral blood circulating tumour cell capture paired with cytomorphologic criteria for the diagnosis of cancer. Lung Cancer. 2014;85:182–5. doi: 10.1016/j.lungcan.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 33.Mascalchi M, Falchini M, Maddau C, Salvianti F, Nistri M, Bertelli E, Sali L, Zuccherelli S, Vella A, Matucci M, Voltolini L, Pegna AL, Luconi M, Pinzani P, Pazzagli M. Prevalence and number of circulating tumour cells and microemboli at diagnosis of advanced NSCLC. J Cancer Res Clin Oncol. 2016;142:195–200. doi: 10.1007/s00432-015-2021-3. [DOI] [PubMed] [Google Scholar]
- 34.Pailler E, Adam J, Barthélémy A, Oulhen M, Auger N, Valent A, Borget I, Planchard D, Taylor M, André F, Soria JC, Vielh P, Besse B, Farace F. Detection of circulating tumor cells harboring a unique ALK rearrangement in ALK-positive non-small-cell lung cancer. J. Clin. Oncol. 2013;31:2273–81. doi: 10.1200/JCO.2012.44.5932. [DOI] [PubMed] [Google Scholar]
- 35.Ilié M, Szafer-Glusman E, Hofman V, Chamorey E, Lalvée S, Selva E, Leroy S, Marquette CH, Kowanetz M, Hedge P, Punnoose E, Hofman P. Detection of PD-L1 in circulating tumor cells and white blood cells from patients with advanced non-small-cell lung cancer. Ann Oncol. 2018;29:193–199. doi: 10.1093/annonc/mdx636. [DOI] [PubMed] [Google Scholar]
- 36.Ilie M, Hofman V, Long E, Bordone O, Selva E, Washetine K, Marquette CH, Hofman P. Current challenges for detection of circulating tumor cells and cell-free circulating nucleic acids, and their characterization in non-small cell lung carcinoma patients. What is the best blood substrate for personalized medicine? Ann Transl Med. 2014;2:107. doi: 10.3978/j.issn.2305-5839.2014.08.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nair VS, Keu KV, Luttgen MS, Kolatkar A, Vasanawala M, Kuschner W, Bethel K, Iagaru AH, Hoh C, Shrager JB, Loo BW Jr, Bazhenova L, Nieva J, Gambhir SS, Kuhn P. An observational study of circulating tumor cells and 18F-FDG PET uptake in patients with treatment-naive non-small cell lung cancer. PLoS One. 2013;8:e67733. doi: 10.1371/journal.pone.0067733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morbelli S, Alama A, Ferrarazzo G, Coco S, Genova C, Rijavec E, Bongioanni F, Biello F, Dal Bello MG, Barletta G, Massollo M, Vanni I, Piva R, Nieri A, Bauckneht M, Sambuceti G, Grossi F. Circulating tumor DNA reflects tumor metabolism rather than tumor burden in chemotherapy-naive patients with advanced non-small cell lung cancer: 18F-FDG PET/CT study. J Nucl Med. 2017;58:1764–1769. doi: 10.2967/jnumed.117.193201. [DOI] [PubMed] [Google Scholar]
- 39.De Giorgi U, Mego M, Rohren EM, Liu P, Handy BC, Reuben JM, Macapinlac HA, Hortobagyi GN, Cristofanilli M, Ueno NT. 18F-FDG PET/CT findings and circulating tumor cell counts in the monitoring of systemic therapies for bone metastases from breast cancer. J Nucl Med. 2010;51:1213–1218. doi: 10.2967/jnumed.110.076455. [DOI] [PubMed] [Google Scholar]
- 40.Delfau-Larue MH, van der Gucht A, Dupuis J, Jais JP, Nel I, Beldi-Ferchiou A, Hamdane S, Benmaad I, Laboure G, Verret B, Haioun C, Copie-Bergman C, Berriolo-Riedinger A, Robert P, Casasnovas RO, Itti E. Total metabolic tumor volume, circulating tumor cells, cell-free DNA: distinct prognostic value in follicular lymphoma. Blood Adv. 2018;2:807–816. doi: 10.1182/bloodadvances.2017015164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abrahamsson J, Aaltonen K, Engilbertsson H, Liedberg F, Patschan O, Rydén L, Sjödahl G, Gudjonsson S. Circulating tumor cells in patients with advanced urothelial carcinoma of the bladder: association with tumor stage, lymph node metastases, FDG-PET findings, and survival. Urol Oncol. 2017;35:606.e9–606.e16. doi: 10.1016/j.urolonc.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 42.Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, Verzijlbergen FJ, Barrington SF, Pike LC, Weber WA, Stroobants S, Delbeke D, Donohoe KJ, Holbrook S, Graham MM, Testanera G, Hoekstra OS, Zijlstra J, Visser E, Hoekstra CJ, Pruim J, Willemsen A, Arends B, Kotzerke J, Bockisch A, Beyer T, Chiti A, Krause BJ European Association of Nuclear Medicine (EANM) FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–354. doi: 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farace F, Massard C, Vimond N, Drusch F, Jacques N, Billiot F, Laplanche A, Chauchereau A, Lacroix L, Planchard D, Le Moulec S, André F, Fizazi K, Soria JC, Vielh P. A direct comparison of CellSearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. Br J Cancer. 2011;105:847–853. doi: 10.1038/bjc.2011.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lecharpentier A, Vielh P, Perez-Moreno P, Planchard D, Soria JC, Farace F. Detection of circulating tumor cells with hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. Br J Cancer. 2011;105:1338–41. doi: 10.1038/bjc.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pailler E, Auger N, Lindsay CR, Vielh P, Islas-Morris-Hernandez A, Borget I, Ngo-Camus M, Planchard D, Soria JC, Besse B, Farace F. High level of chromosomal instability in circulating tumor cells of ROS1-rearranged non-small cell lung cancer. Ann Oncol. 2015;26:1408–1415. doi: 10.1093/annonc/mdv165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chudasawa D, Rice A, Anikin V, Soppa G, Dalal P. Circulating tumor cells in patients with malignant lung tumors undergoing radio-frequency ablation. Anticancer Res. 2015;35:2823–6. [PubMed] [Google Scholar]
- 47.Chudasawa D, Rice A, Soppa G, Anikin V. Circulating tumor cells in patients with lung cancer undergoing endobronchial cryotherapy. Cryobiology. 2015;71:161–163. doi: 10.1016/j.cryobiol.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 48.Fiorelli A, Accardo M, Carelli E, Angioletti D, Santini M, Di Domenico M. Circulating tumor cells in diagnosing lung cancer: clinical and mophological analysis. Ann Thorac Surg. 2015;99:1899–905. doi: 10.1016/j.athoracsur.2014.11.049. [DOI] [PubMed] [Google Scholar]
- 49.Sawabata N, Funaki S, Hyakutake T, Shintani Y, Fujiwara A, Okumura M. Perioperative circulating tumor cells in surgical patients with non-small cell lung cancer: does surgical manipulation dislodge cancer cells thus allowing them to pass into the peripheral blood? Surg Today. 2016;46:1402–1409. doi: 10.1007/s00595-016-1318-4. [DOI] [PubMed] [Google Scholar]
- 50.Illie M, Szafer-Glusman E, Hofman V, Long-Mira E, Suttmann R, Darbonne W, Butori C, Lalvée S, Fayada J, Selva E, Yu W, Marquette CH, Shames DS, Punnoose E, Hofman P. Expression of MET in circulating tumor cells correlates with expression in tumor from advanced-stage lung cancer patients. Oncotarget. 2017;8:26112–26121. doi: 10.18632/oncotarget.15345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mascalchi M, Maddau C, Sali L, Bertelli E, Salvianti F, Zuccherelli S, Matucci M, Borgheresi A, Raspanti C, Lanzetta M, Falchini M, Mazza E, Vella A, Luconi M, Pinzani P, Pazzagli M. Circulating tumor cells and microemboli can differentiate malignant and benign pulmonary lesions. J Cancer. 2017;8:2223–2230. doi: 10.7150/jca.18418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Illie M, Hofman V, Leroy S, Cohen C, Heeke S, Cattet F, Bence C, Lalvée S, Mouroux J, Marquette CH, Hofman P STALKLUNG01 and AIR Study Consortium Investigators. Use of circulating tumor cells in prospective clinical trials for NSCLC patients-standardization of the pre-analytical conditions. Clin Chem Lab Med. 2018;56:980–989. doi: 10.1515/cclm-2017-0764. [DOI] [PubMed] [Google Scholar]
- 53.Frick MA, Kao GD, Aguarin L, Chinniah C, Swisher-McClure S, Berman AT, Levin WP, Cengel KA, DeCesaris C, Hahn SM, Dorsey JF, Simone CB 2nd. Circulating tumor cell assessment in presumed early stage non-small cell lung cancer patients treated with stereotactic body radiation therapy: a prospective pilot study. Int J Radiat Oncol Biol Phys. 2018;102:536–542. doi: 10.1016/j.ijrobp.2018.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lv Q, Gong L, Zhang T, Ye J, Chai L, Ni C, Mao Y. Prognostic value of circulating tumor cells in metastatic breast cancer: a systemic review and meta-analysis. Clin Transl Oncol. 2016;18:322–30. doi: 10.1007/s12094-015-1372-1. [DOI] [PubMed] [Google Scholar]
- 55.Groot Koerkamp B, Rahbari NN, Büchler MW, Koch M, Weitz J. Circulating tumor cells and prognosis of patients with resectable colorectal liver metastases or widespread metastatic colorectal cancer: a meta-analysis. Ann Surg Oncol. 2013;20:2156–65. doi: 10.1245/s10434-013-2907-8. [DOI] [PubMed] [Google Scholar]
- 56.Onstenk W, de Klaver W, de Wit R, Lolkema M, Foekens J, Sleijfer S. The use of circulating tumor cells in guiding treatment decisions for patients with metastatic castrationresistant prostate cancer. Cancer Treat Rev. 2016;46:42–50. doi: 10.1016/j.ctrv.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 57.Liu Y, Ling Y, Qi Q, Lan F, Zhu M, Zhang Y, Bao Y, Zhang C. Prognostic value of circulating tumor cells in advanced gastric cancer patients receiving chemotherapy. Mol Clin Oncol. 2017;6:235–42. doi: 10.3892/mco.2017.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okubo K, Uenosono Y, Arigami T, Mataki Y, Matsushita D, Yanagita S, Kurahara H, Sakoda M, Kijima Y, Maemura K, Natsugoe S. Clinical impact of circulating tumor cells and therapy response in pancreatic cancer. Eur J Surg Oncol. 2017;43:1050–5. doi: 10.1016/j.ejso.2017.01.241. [DOI] [PubMed] [Google Scholar]
- 59.Aggarwal C, Wang X, Ranganathan A, Torigian D, Troxel A, Evans T, Cohen RB, Vaidya B, Rao C, Connelly M, Vachani A, Langer C, Albelda S. Circulating tumor cells as a predictive biomarker in patients with small cell lung cancer undergoing chemotherapy. Lung Cancer. 2017;112:118–25. doi: 10.1016/j.lungcan.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Z, Xiao Y, Zhao J, Chen M, Xu Y, Zhong W, Xing J, Wang M. Relationship between circulating tumour cell count and prognosis following chemotherapy in patients with advanced non-small-cell lung cancer. Respirology. 2016;21:519–25. doi: 10.1111/resp.12696. [DOI] [PubMed] [Google Scholar]
- 61.Tong B, Xu Y, Zhao J, Chen M, Xing J, Zhong W, Wang M. Prognostic significance of circulating tumor cells in non-small cell lung cancer patients undergoing chemotherapy. Oncotarget. 2017;8:86615–24. doi: 10.18632/oncotarget.21255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lopci E, Toschi L, Grizzi F, Rahal D, Olivari L, Castino GF, Marchetti S, Cortese N, Qehajaj D, Pistillo D, Alloisio M, Roncalli M, Allavena P, Santoro A, Marchesi F, Chiti A. Correlation of metabolic information on FDG-PET with tissue expression of immune markers in patients with non-small cell lung cancer (NSCLC) who are candidates to upfront surgery. Eur J Nucl Med Mol Imaging. 2016;43:1954–61. doi: 10.1007/s00259-016-3425-2. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka K, Toyokawa G, Okamoto T, Baba S, Kozuma Y, Matsubara T, Haratake N, Akamine T, Takamori S, Katsura M, Shoji F, Honda H, Oda Y, Maehara Y. Metabolic characteristics of programmed cell death-ligand 1-expression lung cancer on 18F-fluorodeoxyglucose positron emission tomography/computed tomography. Cancer Med. 2017;6:2552–2561. doi: 10.1002/cam4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nygaard AD, Holdgaard PC, Spindler KL, Pallisgaard N, Jakobsen A. The correlation between cell-free DNA and tumour burden was estimated by PET/CT in patients with advanced NSCLC. Br J Cancer. 2014;110:363–368. doi: 10.1038/bjc.2013.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Punnoose EA, Atwal S, Liu W, Raja R, Fine BM, Hughes BG, Hicks RJ, Hampton GM, Amler LC, Pirzkall A, Lackner MR. Evaluation of circulating tumor cells and circulating tumor DNA in non-small cell lung cancer: association with clinical endpoints in a phase II clinical trial of pertuzumab and erlotinib. Clin Cancer Res. 2012;18:2391–2401. doi: 10.1158/1078-0432.CCR-11-3148. [DOI] [PubMed] [Google Scholar]
- 66.Fu L, Zhu Y, Jing W, Guo D, Kong L, Yu J. Incorporation of circulating tumor cells and whole-body metabolic tumor volume of 18F-FDG PET7CT improves prediction of ourcome in IIIB stage small-cell lung cancer. Chin J Cancer Res. 2018;30:596–604. doi: 10.21147/j.issn.1000-9604.2018.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bayarri-Lara CI, de Miguel Pérez D, Cueto Ladrón de Guevara A, Rodriguez Fernández A, Puche JL, Sánchez-Palencia Ramos A, Ruiz Zafra J, Giraldo Ospina CF, Delgado-Rodríguez M, Expósito Ruiz M, Moyano Rodriguez MJ, Lorente JA, Serrano MJ. Association of circulating tumour cells with early relapse and 18F-fluorodeoxyglucose positron emission tomography uptake in resected non-small-cell lung cancers. Eur J Cardiothorac Surg. 2017;52:55–62. doi: 10.1093/ejcts/ezx049. [DOI] [PubMed] [Google Scholar]


