Fig. 2.
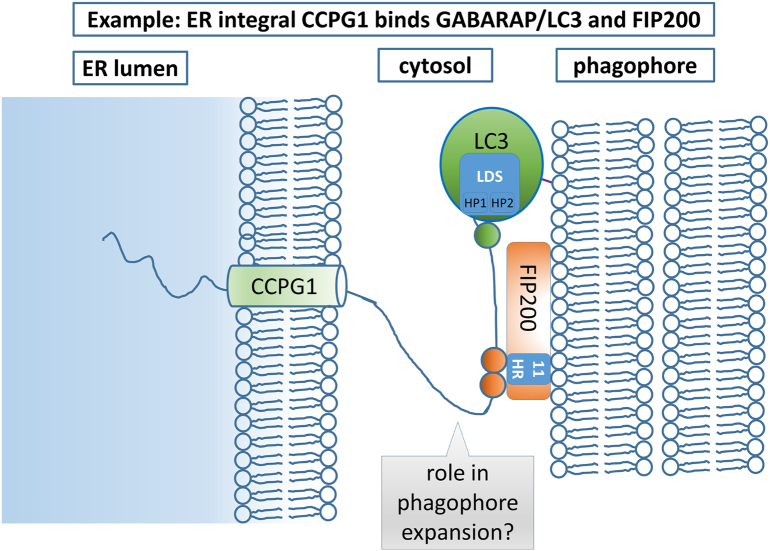
Principle 1: Membrane integral receptor proteins bind to the phagophore. This is exemplified here by consideration of the mammalian cargo receptor CCPG1, which illustrates a number of key points. It is embedded in the single phospholipid bilayer of the ER (membrane on left hand side of cartoon) via a single transmembrane domain. It also binds to ATG proteins assumed resident on the phagophore (growing double phospholipid bilayer, right-hand side of cartoon). First, lipid-conjugated GABARAP/LC3 family members contain a LIR docking site (LDS), which is home to the two hydrophobic pockets (HP1 and HP2) that accommodate the hydrophobic LIR motif on CCPG1 (green circle). All known ER-phagy cargo receptors contain one or more LIR motifs for binding GABARAP/LC3 (or AIM motifs for binding Atg8 in yeast). A second interaction, seen with only some cargo receptors such as CCPG1 or Atg39, is direct binding of a FIR motif(s) (orange circles) to the Atg11 homology region (Atg11HR) at the C-terminus of FIP200 (the Atg11BR, or Atg11-binding region, of yeast Atg39 binds to the autophagy protein Atg11 in an analogous interaction). CCPG1 is depicted here as having two discrete FIR motifs interacting with one molecule of FIP200, but it must be noted that the precise structural details of this interaction, and definition of what constitutes a single mammalian FIR motif, are not yet known. The cytosolic region of CCPG1 is intrinsically disordered, potentially allowing sufficient distance between the GABARAP/LC3 and/or FIP200 interaction sites from the outer leaflet of the ER to avoid steric hindrance (as experimentally demonstrated for TEX264). In principle, receptors need not be ER membrane integral proteins but could form a complex with integral proteins. However, the best-characterized receptors to date (Fig. 1) are all anchored in the ER membrane. Minimally, receptors link cargo to the phagophore or growing autophagosome. However, it is emerging that some, particular those that bind FIP200 and thus potentially the ULK complex, such as CCPG1, might also influence the formation or growth of the phagophore. See “Function and interplay of ATG protein interactions” for further discussion of this.