Fig. 4.
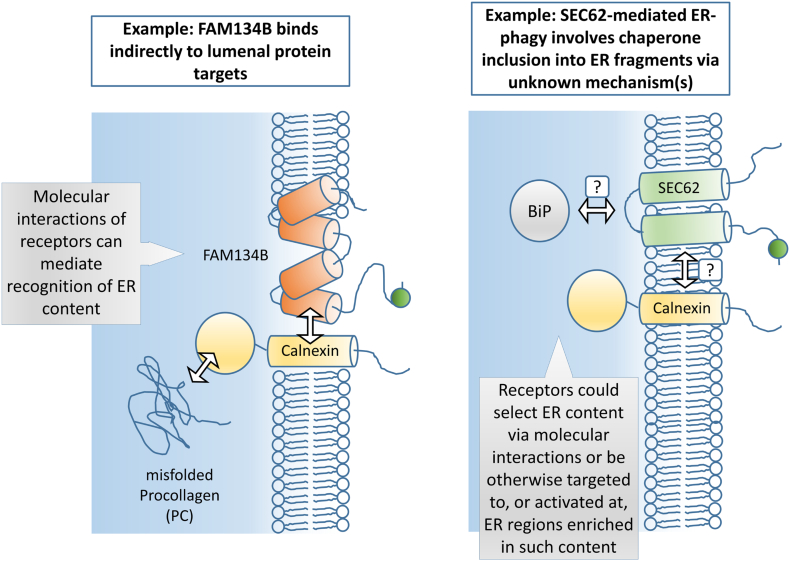
Principle 3: Selection of specific content from within the ER network for degradation. Selective degradation of ER content by ER-phagy may occur because receptors are recruited to the pathway at particular subER locales. Alternatively, and not mutually exclusively with such a mechanism, FAM134B provides an example of how molecular interactions can bridge receptors to lumenal cargo that is to be cleared preferentially from within the ER. Interaction of the RHD (orange cylinders) of FAM134B with the transmembrane domain of Calnexin enables indirect interaction of FAM134B with misfolded procollagen (PC), via the chaperone domain of Calnexin (yellow circle). Thus, FAM134B-driven ER-phagy can be biased toward fragments of ER that are heavily enriched in PC. In principle, other receptors, dependent on their domain structure, could participate in direct or indirect interactions with specific ER protein species that are localized either in the membrane or in the lumen. However, this area of ER-phagy study is in its infancy. In an alternative example, where the molecular mechanism is largely unclear, SEC62 promotes clearance of ER fragments enriched in UPR-upregulated chaperones, such as the integral membrane protein Calnexin and the lumenal protein BiP (Binding immunoglobulin protein). It is unclear whether SEC62 is activated locally, at regions where these cargo molecules accumulate (for example by loss of interaction with SEC63, see “ER-phagy is regulated by cellular state and signal transduction”), or whether SEC62 directly or indirectly interacts with the protein targets. These mechanisms are not mutually exclusive.