Figure 3.
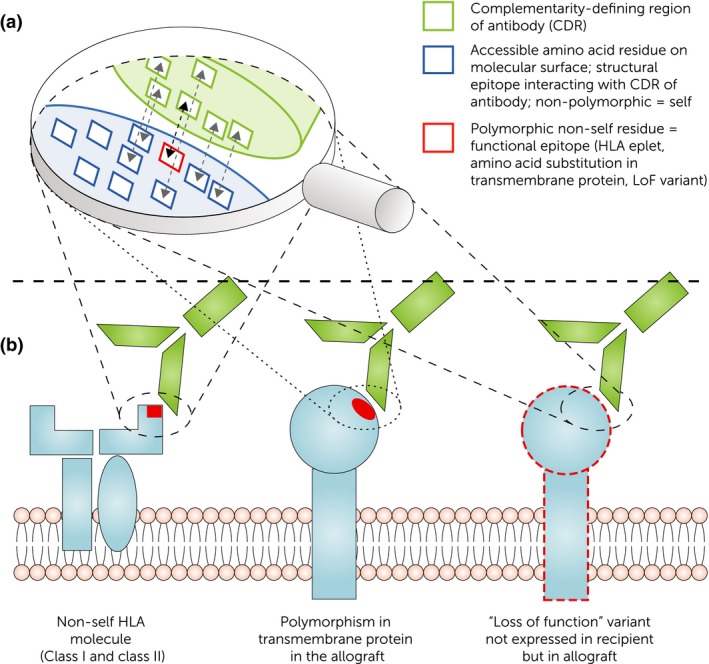
B cell epitopes recognized by donor‐specific antibodies on alloantigens. (a) Interaction of complementarity‐determining region (CDR) of alloantibody with the HLA and non‐HLA molecules on endothelial cells. Next to the functional epitope responsible for specificity (e.g., eplet, amino acid substitution in transmembrane protein, loss of function variant carrying a non‐self epitope), the structural epitope covers non‐polymorphic (“self”) residues important for binding strength/affinity. (b) Concept of non‐self epitopes on HLA and non‐HLA antigens as binding site for donor‐specific alloantibodies (DSA). HLA‐DSA binding to polymorphic residue on HLA molecule. Non‐HLA‐DSA recognizing non‐self residues on polymorphic transmembrane proteins as well as non‐self residues on loss of function variants (i.e., recipient has complete loss of gene expression of a specific allele but the donor carries at least one functioning copy).
