Figure 2.
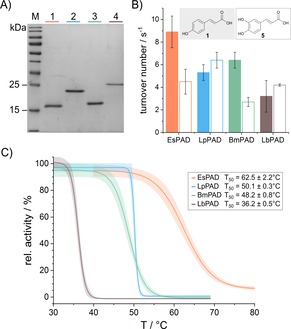
A) EsPAD (20 kDa, lane 1), LpPAD (22 kDa, lane 2), BmPAD (20 kDa, lane 3) and LbPAD (23 kDa, lane 4) were produced as pure enzymes, as indicated by the Coomassie stained 15 % SDS tris‐glycine polyacrylamide gel. M: PageRuler Prestained Protein Ladder (Thermo Scientific). B) Enzymatic activity of all four PAD enzymes at 28 °C with p‐coumaric acid (1) (filled bars) and caffeic acid (5) (empty bars) as substrate. C) Average thermal inactivation curves with 95 % confidence interval of all four purified PAD enzymes after 10 min incubation and their corresponding T50 values (for individual data sets see Figures S2–S5). Analyses B and C were carried out in technical duplicates and biological triplicates.
