Figure 1.
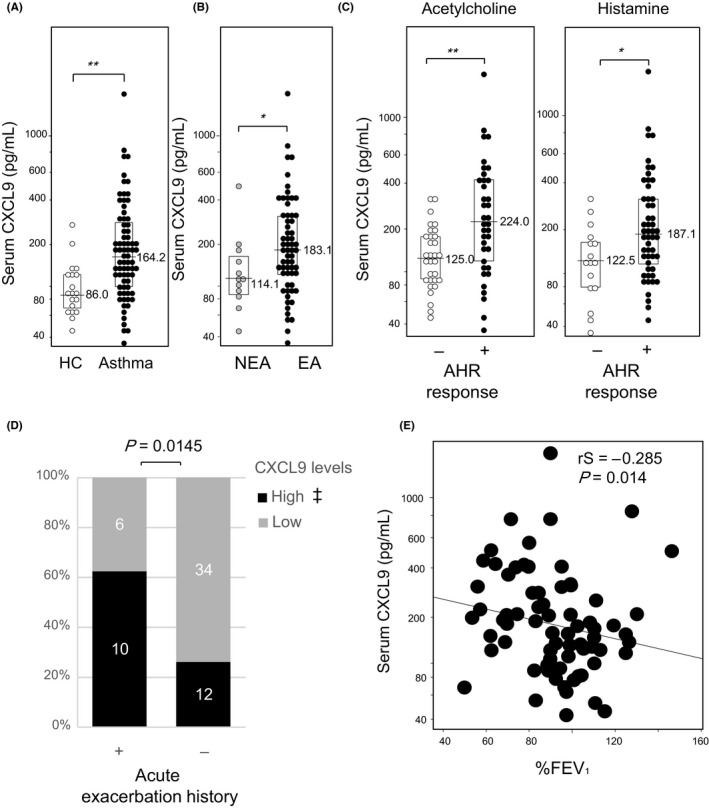
Serum CXCL9 concentrations reflect clinical characteristics of patients with asthma. A, Healthy controls (HC, open) and asthma (closed); B, eosinophilic asthma (EA, closed) and noneosinophilic asthma (NEA, gray); C, patients who showed more than (closed) or less than (open) 20% decrease in FEV1 up to 20 mg/mL acetylcholine (left) or up to 10 mg/mL histamine (right) challenge tests were defined as AHR‐positive or AH‐negative, respectively. Results are presented as individual data points with medians (bars) and interquartile ranges (boxes). Median values are indicated next to the bars. P‐values were calculated by the Mann‐Whitney U test. *: P < 0.05, **: P < 0.005; D, Relation between CXCL9 levels and acute exacerbation history; P‐value was calculated by Fisher's exact test. Exacerbation was defined based on systemic steroid usage within one year after blood collection. ‡: Cutoff value was defined by 2SD of HC distribution (229 pg/mL). E, Correlation was analyzed between %FEV1 and CXCL9 concentrations by Spearman's rank correlation
