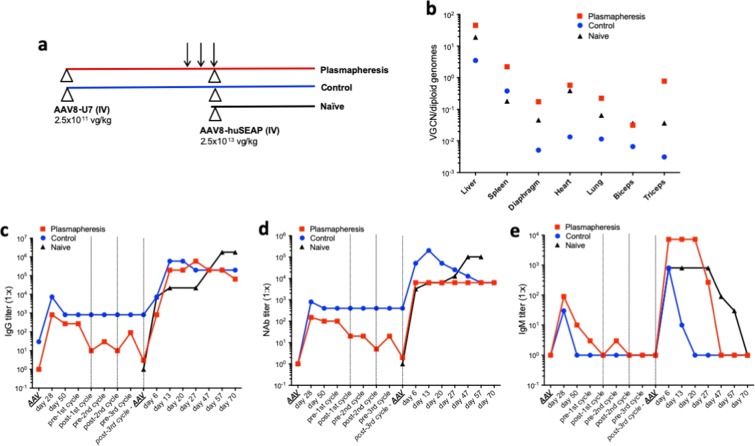
Figure 1.
Plasmapheresis allows for AAV vector readministration in non-human primates. (a) Experimental design. Two animals received an AAV8-U7 vector intravenously at a dose of 2.5 × 1011vg/kg (Plasmapheresis and Control), 6 weeks later one animal (Plasmapheresis) was subjected to 3 cycles of plasmapheresis at a 2-day interval (arrows). Both animals and a third naïve control animal (Naïve) were then infused with an AAV8-huSEAP vector at a dose of 2.5 × 1013 vg/kg. Triangles indicate AAV vector administrations. (b) Vector genome copy number (VGCN) per diploid genome in various tissues collected at sacrifice. (c–e) Anti-AAV8 IgG (c), NAb (d), and IgM (e) levels over time. AAV in the x axis indicate the timing of AAV vector administration. Vertical dotted lines represent the timing of plasmapheresis cycles. Titers are reported as 1:x dilution.