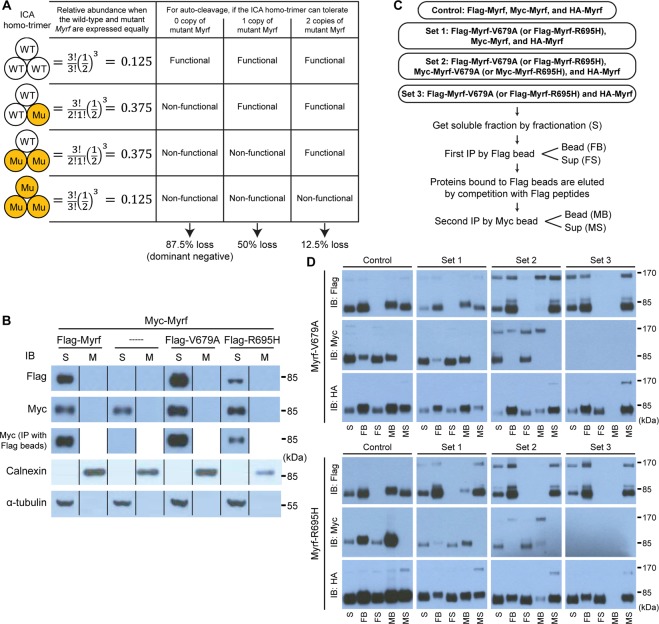
Figure 5.
Fractionation and immunoprecipitation of wild-type and mutant Myrf N-terminal fragments. (A) The relative abundance of distinct ICA homo-trimer species when wild-type and mutant Myrf proteins are present in equal abundance. The fraction of disabled ICA homo-trimers depending on the number of the mutant Myrf tolerated by the ICA homo-trimer for its auto-cleavage function. (B) The auto-cleavage of mutant Myrf when complexed with two copies of wild-type Myrf. Myc-Myrf was transfected into HEK293FT cells, either alone (-----) or together with Flag-Myrf, Flag-Myrf-V679A, or Flag-Myrf-R695H. Whole cell lysates were separated into soluble (“S”) and membrane (“M”) fractions by fractionation. Immunoblotting with calnexin (an ER membrane protein) and α-tubulin indicated a good separation (the bottom two rows). The M and S fractions were blotted for Myrf N-terminal fragments with Flag and Myc antibodies (the top two rows). To determine physical association between wild-type and mutant Myrf N-terminal fragments, the S fractions were subject to immunoprecipitation with Flag beads, which were blotted for Myc-Myrf N-terminal fragments with Myc antibodies (the middle row). IP: immunoprecipitation. IB: immunoblotting. These samples were run on the same gel, cut, and put together. The grouping of blots cropped from different parts of the same gel is indicated by dividing lines. The raw Western blot results are available in Fig. S8 (Flag), Fig. S9 (Myc), Fig. S10 (calnexin), and Fig. S11 (α-tubulin), where the cropped portions are marked by yellow boxes. (C) Sequential immunoprecipitation to determine the maximum number of Myrf-V679A or Myrf-R695H that is tolerated by the ICA homo-trimer for its auto-cleavage function. For each mutant Myrf (Myrf-V679A and Myrf-R695H), 4 sets of plasmids were expressed in HEK293FT cells. After cells were broken by homogenization, the soluble fraction was obtained by fractionation (“S”). It was subject to immunoprecipitation with Flag beads, yielding the bead and sup fractions (“FB” and “FS”). Proteins bound to Flag beads were eluted by competition with Flag peptides. The eluate was subject to another round of immunoprecipitation with Myc beads, yielding the bead and sup fractions (“MB” and “MS”). (D) The five fractions (“S”, “FB”, “FS”, “MB”, and “MS”) from the sequential immunoprecipitation were blotted for full-length Myrf and Myrf N-terminal fragments with Flag, Myc, and HA antibodies. For each of the 6 immunoblots, the loading ratio among the five fractions was kept constant across the four sets for an objective comparison. IB: immunoblotting.