Figure 1.
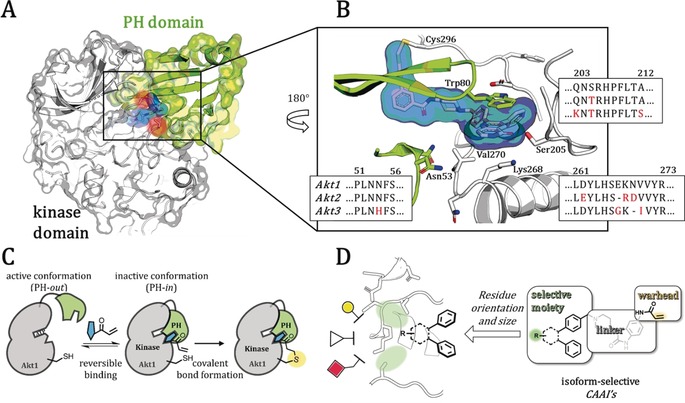
Covalent‐allosteric Akt inhibitors as a beneficial starting point to serve as tools for investigation of isoform selectivity. A) Co‐crystal structure of full‐length Akt1 in complex with a covalent‐allosteric inhibitor (highlighted in blue, PDB: 6HHI) shows covalent binding to one of two conserved cysteines (red) and stabilizes an inactive conformation through interaction with the kinase domain (white) and the PH‐domain (green). B) Detailed view of the interdomain binding pocket in comparison with an Akt‐isoform sequence alignment (with BLAST); alterations of amino acids (red). C) General scheme of the conformational changes upon allosteric ligand binding; first a reversible step takes place followed by the irreversible modification. D) Design of covalent‐allosteric ligands, which contain different moieties and orientations towards the binding pocket part where amino acid alterations in the Akt‐isoforms occur.
