Figure 3.
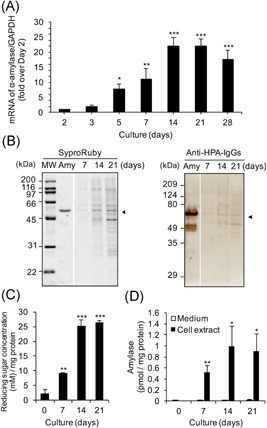
Expression of α‐amylase in Caco‐2 cells cultured 28 days. The cells were seeded at 5 × 104 cells/cm2. The first medium change was made 2 days after seeding, and then the medium was changed every 2 to 3 days until 21 or 28 days. A, mRNA expression of α‐amylase: The cells were seeded and cultured in six‐well plates. The expression of α‐amylase was determined by real‐time PCR. The expression for days 3 to 28 (ΔΔC t method vs that cultured for day 2) is shown; mean ± SE for 4 to 5 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 vs day 2 by one‐way ANOVA with Dunnett's post‐hoc test. B, Western blot analysis for α‐amylase: The cells were seeded and cultured in a dish (100 × 20 mm). The cell extracts were boiled with SDS‐PAGE sample buffer ×5 including 2‐mercaptoethanol, and aliquots (10 μL each) were used for Western blot analysis as described in Section 2, left. Protein staining using Sypro Ruby, right. Immunostaining for α‐amylase using rabbit anti‐HPA IgGs and HRP‐conjugated goat anti‐rabbit IgGs. MW denotes molecular weight markers, Amy is purified pig pancreatic α‐amylase (0.8 μg/lane), 7 to 21 days: Extract of cells cultured for 7, 14, or 21 days (1.4, 2.4, and 3.7 μg protein/lane each). C–D, Enzymatic activity of α‐amylase: The cells were seeded and cultured in 24‐well plates for 7 to 21 days. The cell extracts were assayed for (C) starch degrading activity (1 vol was 10 μL) and (D) α‐amylase activity as described in Section 2. The activity for days 7 to 21 is shown as mean ± SE for three independent experiments. ANOVA, analysis of variance; HPA, human pancreatic α‐amylase; HRP, horseradish peroxidase; IgG, immunoglobulin G; mRNA, messenger RNA; MW, molecular weight; SDS‐PAGE, sodium dodecyl sulfate‐polyacrylamide gel electrophoresis; □, medium; ■, cell extract. (C) **P < 0.01, ***P < 0.001 vs day 0 by one‐way ANOVA with Dunnett's post‐hoc test. (D) *P < 0.05, **P < 0.01 vs medium by paired t test
