Abstract
OBJECTIVE
To confirm efficacy and safety of fast-acting insulin aspart (faster aspart) versus insulin aspart (IAsp), both with basal insulin degludec, in a pediatric population with type 1 diabetes.
RESEARCH DESIGN AND METHODS
After a 12-week run-in, this treat-to-target, 26-week, multicenter trial randomized participants (1 to <18 years) to double-blind mealtime faster aspart (n = 260), mealtime IAsp (n = 258), or open-label postmeal faster aspart (n = 259). The primary end point was change from baseline in glycated hemoglobin (HbA1c) after 26 weeks of treatment. All available information regardless of treatment discontinuation was used for the evaluation of treatment effect.
RESULTS
At week 26, mealtime and postmeal faster aspart were noninferior to IAsp regarding change from baseline in HbA1c (P < 0.001 for noninferiority [0.4% margin]), with a statistically significant difference in favor of mealtime faster aspart (estimated treatment difference −0.17% [95% CI −0.30; −0.03], −1.82 mmol/mol [−3.28; −0.36]; P = 0.014). Change from baseline in 1-h postprandial glucose increment significantly favored mealtime faster aspart versus IAsp at breakfast, main evening meal, and over all meals (P < 0.01 for all). No statistically significant differences in the overall rate of severe or blood glucose–confirmed hypoglycemia were observed. Mean total daily insulin dose was 0.92 units/kg for mealtime faster aspart, 0.92 units/kg for postmeal faster aspart, and 0.88 units/kg for mealtime IAsp.
CONCLUSIONS
In children and adolescents with type 1 diabetes, mealtime and postmeal faster aspart with insulin degludec provided effective glycemic control with no additional safety risks versus IAsp. Mealtime faster aspart provided superior HbA1c control compared with IAsp.
Introduction
Type 1 diabetes is a common chronic disease of childhood and adolescence (1,2). In 2017, more than 1,106,500 children were living with type 1 diabetes globally, and this number is predicted to rise (3). Although guidelines for management of children and adolescents with type 1 diabetes have lowered glycated hemoglobin (HbA1c) targets from <7.5% to as low as ≤6.5% (4–7), findings from a clinical registry suggest that the majority of patients are not meeting the <7.5% treatment target (8).
Intensive insulin regimens are recommended to achieve glycemic targets in children and adolescents with type 1 diabetes (4,5), resulting in better glycemic control and reduced risk of chronic complications (9–11). However, challenges associated with insulin therapy in this age-group include marked postmeal glycemic excursions (12). Although the increased use of basal-bolus regimens helps to improve HbA1c levels in children (4), there remains an unmet need for faster-acting mealtime insulins that more closely mimic endogenous prandial insulin action, as absorption kinetics of currently available options (insulin lispro, insulin aspart [IAsp], and insulin glulisine) are inadequate to achieve optimal postprandial glucose (PPG) control (5,13–15).
Fast-acting insulin aspart (faster aspart) is a new formulation of IAsp containing the added excipients niacinamide and l-arginine. Findings in children and adolescents demonstrated that after injection, onset of appearance occurred approximately twice as fast (5–7 minutes earlier) and the early glucose-lowering effect was 78–147% greater for faster aspart compared with IAsp (16). In terms of glycemic control in adults with type 1 diabetes, mealtime and postmeal faster aspart, in combination with either insulin detemir or insulin degludec, have consistently been found to be noninferior to mealtime IAsp in terms of change in HbA1c, with significant improvements in PPG control with mealtime administration (17,18). Furthermore, the reduction in HbA1c with mealtime faster aspart in combination with insulin detemir was statistically significantly different versus mealtime IAsp (18).
The purpose of the onset 7 trial was to evaluate the efficacy and safety profile of faster aspart administered at mealtime or postmeal compared with mealtime IAsp, both combined with insulin degludec, in children and adolescents with type 1 diabetes. The trial was designed to quantify a population average effect for participants with type 1 diabetes irrespective of adherence to randomized treatment and use of ancillary therapies.
Research Design and Methods
Trial Design
This 26-week, phase 3, multicenter, randomized, double-blind, parallel-group trial (ClinicalTrials.gov, reg. no. NCT02670915) compared the efficacy and safety of mealtime faster aspart versus mealtime IAsp, both in combination with insulin degludec, in children and adolescents with type 1 diabetes. The trial also included an open-label treatment arm with participants receiving postmeal faster aspart in combination with insulin degludec (Supplementary Fig. 1). The trial included 150 sites across 17 countries (Supplementary Data). Randomization was stratified by age-group (≥1 to <3 years, ≥3 to <6 years, ≥6 to <12 years, and ≥12 to <18 years) based on participant age at randomization. Follow-up occurred 7 and 30 days after the end of treatment. The trial was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization of Good Clinical Practice.
Study Population
Eligible participants were aged 1 to <18 years at randomization (2 to <18 years in Serbia) with type 1 diabetes and receiving basal-bolus treatment (using a basal analog or NPH insulin) for ≥90 days prior to screening. Participants were required to have an HbA1c ≤9.5% (80 mmol/mol) and be willing to not use real-time continuous glucose monitoring (CGM) during the trial.
A subgroup of participants at selected sites used a blinded CGM and received two standardized meal tests during the trial. Eligible participants were ≥8 years of age at screening and had demonstrated an ability and willingness to use the flexible bolus dosing principles based on carbohydrate counting, as judged by the investigator.
Exclusion
Participants were excluded if they had known or suspected hypersensitivity to trial products or related products, anticipated initiation or change in concomitant medication for more than 14 days known to affect weight or glucose metabolism (e.g., orlistat, thyroid hormones [treated hypothyroidism allowed], or corticosteroids), had received treatment with any medication for the indication of diabetes or obesity other than stated in the inclusion criteria in the 90 days before screening, or experienced more than one episode of diabetic ketoacidosis requiring hospitalization within the last 90 days prior to screening. Females who were pregnant, planning to become pregnant, or breastfeeding were excluded (see Supplementary Data for full criteria).
Treatment Interventions
Basal Insulin
After a 2-week screening period, participants underwent a 12-week run-in, during which time participants switched from their previous basal insulin to insulin degludec (100 units/mL, 3-mL pen injector) with dose optimization based on protocol-specified guidelines (Supplementary Table 1). Insulin degludec was administered once daily at any time of day but preferably at the same time each day. During run-in, the dose was titrated weekly by the investigator to a prebreakfast target of 4.0–8.0 mmol/L (71–145 mg/dL), with any further adjustments made at the investigator’s discretion.
Bolus Insulin
During run-in, all participants switched to IAsp (with a calculated basal/bolus ratio between 50:50 and 30:70), and the dose was adjusted only when considered necessary by the investigator. After run-in, participants with HbA1c ≤9.5% (≤80 mmol/mol) were randomized 1:1:1 to receive double-blind mealtime faster aspart, double-blind mealtime IAsp, or open-label postmeal faster aspart.
Bolus insulin (faster aspart or IAsp supplied as 100 units/mL, 3-mL pen injector with 0.5-unit increments) was administered at each of the three main meals (i.e., breakfast, lunch, and main evening meal). Mealtime doses were injected 0–2 min before the meal, whereas postmeal doses were injected 20 min after the start of the meal. Additional bolus dosing was allowed at the investigator’s discretion.
During the 26-week treatment period, bolus insulin was titrated to a preprandial target of 4.0–8.0 mmol/L (71–145 mg/dL) and a bedtime target of 6.7–10 mmol/L (120–180 mg/dL) in a treat-to-target approach. Participants (along with parents/guardians) dosed bolus insulin using flexible dosing principles based on carbohydrate counting or using a predefined bolus-dosing algorithm (Supplementary Table 2). For participants adequately trained in the principles of flexible bolus dosing, bolus dose adjustment was conducted several times daily in accordance with insulin-to-carbohydrate ratios and a plasma glucose correction factor. For participants using the predefined bolus-dosing algorithm (Supplementary Table 2), bolus dose titration was performed once weekly based on the lowest premeal and bedtime plasma glucose values measured on the 3 days prior to a site visit/contact.
Self-Measured Blood Glucose
Participants were supplied with a blood glucose (BG) meter (Abbott Precision Neo or Precision; Abbott Laboratories, Chicago, IL), factory calibrated to display plasma equivalent glucose values, and were instructed to record date, time, and value of all self-measured blood glucose (SMBG) measurements relating to four- and eight-point profiles and hypoglycemic episodes. Four-point profiles (before each main meal [breakfast, lunch, and main evening meal] and bedtime) were recorded daily for insulin titration purposes. Eight-point profiles were recorded on the two consecutive days prior to visits at weeks 0, 12, and 26 (protocol described in Supplementary Table 3).
CGM and Meal Test
Participants in the CGM subgroup used a blinded device (Dexcom G4) for 11–13 days before randomization and from 11 to 13 days before the end of the 26-week treatment period. Participants were instructed to use the CGM according to the manufacturer’s guidelines regarding calibration. This subgroup had two standardized liquid-meal tests in connection with CGM wearing at baseline (week 0) and at end of treatment (week 26). Test meals were avoided on the 1st and 7th day after sensor insertion.
Standardized Meal Test
Participants attended the meal test in a fasted state with a plasma glucose of 4.0–8.8 mmol/L (71–160 mg/dL). Before randomization at baseline (week 0), a bolus dose of IAsp was administered (calculated by dividing carbohydrate content by the participant’s insulin-to-carbohydrate ratio), followed by ingestion of a standardized liquid meal (1.5 g carbohydrates/kg body weight at baseline, to a maximum of 80 g of carbohydrates). Blood samples were taken immediately before the meal test (−2 min) and after 30 min, 1 h, and 2 h (0 h defined as the start of meal consumption). The liquid-meal test was repeated at week 26 using the participant’s study medication and the same insulin dosing and carbohydrate consumption as at baseline. Participants randomized to postmeal dosing received their bolus dose 20 min after starting the last meal test.
End Points
The primary end point was change from baseline in HbA1c 26 weeks after randomization. Key supportive secondary end points included change from baseline to week 26 in the following: PPG and PPG increment (eight-point SMBG profile), mean of the eight-point SMBG profile, fasting plasma glucose (FPG), and 1,5-anhydroglucitol (1,5-AG). Key supportive secondary safety end points included treatment-emergent adverse events, treatment-emergent hypoglycemic episodes, change in body weight, and insulin dose. A nonexclusive list of predefined end points is provided in Supplementary Table 4.
Statistical Methods
All primary and secondary efficacy end points were summarized and analyzed using the full analysis set, unless otherwise stated. Safety end points were summarized using the safety analysis set and analyzed using the full analysis set, unless otherwise stated. All efficacy end points, except insulin dose, were assessed using the in-trial observation period and repeated using the on-treatment observation period. Insulin dose was only presented using the on-treatment observation period.
The primary objective of the trial was to confirm the effect of treatment with mealtime faster aspart in terms of glycemic control measured by change from baseline in HbA1c 26 weeks after randomization by comparing it to treatment with mealtime IAsp, both in combination with insulin degludec, using a noninferiority approach in children and adolescents with type 1 diabetes. The trial also aimed to confirm the effect of treatment with postmeal faster aspart as measured by change from baseline in HbA1c 26 weeks after randomization and to confirm superiority of mealtime faster aspart versus IAsp. This was done following a stepwise hierarchical procedure (Supplementary Tables 5 and 6). Noninferiority (primary end point) was confirmed if the upper boundary of the two-sided 95% CI was ≤0.4%. The sample size was determined to ensure sufficient power for the first step and the second step in the hierarchical testing procedure.
Change in HbA1c from baseline to 26 weeks was analyzed with a statistical model using multiple imputations where participants with missing data at scheduled visits had their HbA1c values imputed using available information from the treatment arm to which the participant had been randomized. A similar statistical model, but with the corresponding baseline value as covariate, was used to analyze change from baseline to 26 weeks in PPG and PPG increments and mean of the eight-point profile. The multiple imputation model was also used to analyze FPG and 1,5-AG, except with baseline FPG and baseline 1,5-AG as covariates, respectively. End points related to the meal test and CGM were analyzed using an ANOVA model, including treatment, region, and strata (age-group at randomization [≥1 to <3 years, ≥3 to <6 years, ≥6 to <12 years, and ≥12 to <18 years]) as factors and the corresponding baseline values as covariate. The number of treatment-emergent severe or BG-confirmed hypoglycemic episodes (all, daytime, and nocturnal), as well as treatment-emergent severe or BG-confirmed hypoglycemic episodes in relation to time since start of meal with time intervals up to 4 h was analyzed using a negative binomial regression model.
For end points summarized by age subgroup, the ≥1 to <3 and ≥3 to <6 years age-groups were merged before database lock due to the small number of participants <3 years of age. Further details on the statistical methods for the primary and secondary end points, the observation periods, and the sample size calculation are provided in the Supplementary Data.
Results
Trial Participants
Participants (n = 777) were randomized to mealtime faster aspart (n = 260), mealtime IAsp (n = 258), or postmeal faster aspart (n = 259), and all were exposed to study medication. A total of 760 participants (97.8%) completed the trial, and 756 participants (97.3%) completed the 26-week treatment period without premature discontinuation of randomized treatment (Supplementary Fig. 2). The number of participants who withdrew from the trial or discontinued randomized treatment was distributed similarly across treatment arms (Supplementary Fig. 2). Baseline demographic and disease characteristics were similar between the three treatment arms (Table 1). In total, 464 participants (60.0%) used flexible dosing principles based on carbohydrate counting at baseline, with a similar number of participants in each treatment arm.
Table 1.
Baseline characteristics
| Faster aspart (mealtime), n = 260 | Faster aspart (postmeal), n = 259 | IAsp (mealtime), n = 258 | |
|---|---|---|---|
| Sex, male, n (%) | 134 (51.5) | 137 (52.9) | 148 (57.4) |
| Age, n (%) | 11.7 (3.7) | 11.6 (3.7) | 11.7 (3.4) |
| ≥1 and <6 years | 16 (6.2) | 16 (6.2) | 14 (5.4) |
| ≥6 and <12 years | 100 (38.5) | 100 (38.6) | 101 (39.1) |
| ≥12 and <18 years | 144 (55.4) | 143 (55.2) | 143 (55.4) |
| BMI (kg/m2) | 19.7 (3.8) | 19.7 (4.0) | 19.6 (3.8) |
| Diabetes duration (years) | 4.5 (3.5) | 4.4 (3.2) | 4.3 (3.1) |
| HbA1c (%) | 7.6 (0.8) | 7.6 (0.8) | 7.5 (0.8) |
| mmol/mol | 59.3 (8.7) | 59.4 (9.1) | 58.8 (9.1) |
| FPG central lab (mmol/L) | 7.6 (3.6) | 8.0 (3.4) | 7.8 (3.5) |
| mg/dL | 136.7 (64.2) | 144.6 (60.3) | 140.4 (62.7) |
| Carbohydrate counting, n (%) | 152 (58.5) | 156 (60.2) | 156 (60.5) |
Results are presented as arithmetic means (SD) unless otherwise stated.
Efficacy
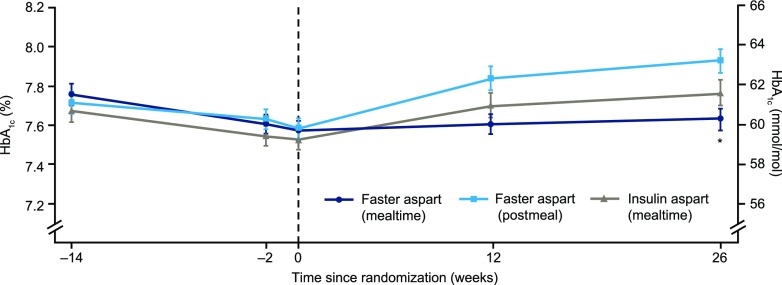
Change in HbA1c during run-in and treatment periods is described in Fig. 1. Mealtime and postmeal faster aspart were both noninferior to mealtime IAsp with regard to change from baseline in HbA1c 26 weeks after randomization (estimated treatment difference [ETD] mealtime −0.17% [95% CI −0.30; −0.03], −1.82 mmol/mol [−3.28; −0.36]; postmeal 0.13% [−0.01; 0.26], 1.40 mmol/mol [−0.06; 2.86]; one-sided P < 0.001 for noninferiority). Change from baseline in HbA1c with mealtime faster aspart was superior to mealtime IAsp (P = 0.007).
Figure 1.
Mean HbA1c over time. During run-in, observed mean HbA1c was reduced from 7.8% (61.3 mmol/mol) to 7.6% (59.3 mmol/mol) for participants subsequently randomized to mealtime faster aspart, from 7.7% (60.4 mmol/mol) to 7.5% (58.8 mmol/mol) for those randomized to mealtime IAsp, and from 7.7% (60.8 mmol/mol) to 7.6% (59.4 mmol/mol) for those randomized to postmeal faster aspart. At the end of the 26-week treatment period, mean HbA1c was 7.6% (59.9 mmol/mol), 7.8% (61.3 mmol/mol), and 7.9% (63.0 mmol/mol) in the mealtime faster aspart, mealtime IAsp, and postmeal faster aspart arms, respectively. Faster aspart (mealtime): IAsp (mealtime) ETD at week 26 –0.17% (95% CI −0.30; −0.03), *P = 0.014. Faster aspart (postmeal): IAsp (mealtime) ETD at week 26 0.13% (95% CI –0.01; 0.26), P = 0.061. Noninferiority of mealtime and postmeal faster aspart versus mealtime IAsp confirmed (P value from the one-sided test for noninferiority with 0.4% margin evaluated at the 2.5% level: P < 0.001). Error bars: ±SE. All available information regardless of treatment discontinuation was used.
No considerable differences in change from baseline in HbA1c were observed between treatment arms across different age subgroups (Supplementary Fig. 3). In an exploratory post hoc analysis, the change from baseline in HbA1c was independent of age across treatments (Supplementary Fig. 4).
The odds of achieving HbA1c <7.5% (58.5 mmol/mol) were not statistically significantly different with mealtime or postmeal faster aspart compared with mealtime IAsp (Supplementary Table 7).
SMBG
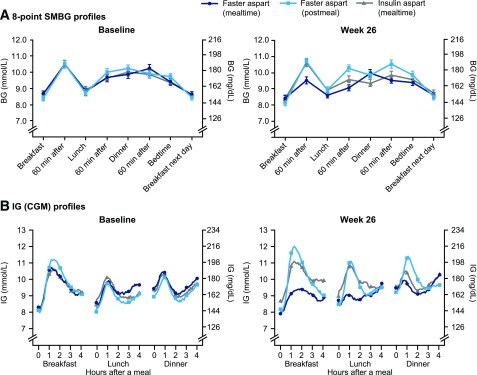
The eight-point SMBG profiles at baseline and week 26 are shown in Fig. 2A. No statistically significant difference in mean SMBG was observed with mealtime or postmeal faster aspart compared with mealtime IAsp (Supplementary Table 7). Change from baseline in 1-h PPG at breakfast, lunch, and over all meals was statistically significantly in favor of mealtime faster aspart versus mealtime IAsp (Supplementary Table 7). Change from baseline in 1-h PPG at lunch, main evening meal, and over all meals was statistically significantly in favor of mealtime IAsp versus postmeal faster aspart (Supplementary Table 7). With regard to change from baseline in 1-h PPG increment, the ETDs (95% CI) were statistically in favor of mealtime faster aspart at breakfast, main evening meal, and over all meals versus mealtime IAsp, and statistically in favor of mealtime IAsp only over all meals (and not for any individual meals) versus postmeal faster aspart (Supplementary Table 7).
Figure 2.
Glucose profiles at baseline and 26 weeks after randomization with SMBG (A) and CGM (B). Error bars: ±SE in A. SMBG profiles are plasma equivalent glucose values.
CGM and Meal-Test Subgroup Results
The baseline characteristics of this subgroup (n = 135) were similar across treatment arms (Supplementary Table 8). Mean prandial interstitial glucose (IG) profiles by individual meal at baseline and week 26 are shown in Fig. 2B. Results of the statistical analysis of the mean IG increments are shown in Supplementary Fig. 5. Change from baseline in 1-h and 2-h IG increment 26 weeks after randomization was statistically significantly in favor of mealtime faster aspart versus mealtime IAsp at breakfast, main evening meal, and over all meals (Supplementary Table 7), similar to the findings of the 1-h postprandial SMBG profiles. Change from baseline in 1-h IG increment was statistically significantly in favor of mealtime IAsp versus postmeal faster aspart over all meals (but not for any individual meal). Change in 2-h IG increment was statistically significantly in favor of mealtime IAsp versus postmeal faster aspart for breakfast, lunch, and over all meals (Supplementary Table 7).
There was a statistically significantly greater reduction from baseline in mean IG peak after start of meal with mealtime faster aspart versus mealtime IAsp at breakfast (ETD −1.29 mmol/L [95% CI −2.22; −0.35], −23.19 mg/dL [−40.02; −6.36]; P = 0.007) and lunch (−1.00 mmol/L [−1.91; −0.08], −17.96 mg/dL [−34.43; −1.49]; P = 0.033), but no statistically significant changes were observed after the main evening meal or across the mean of all meals; no statistical differences were reported between postmeal faster aspart and mealtime IAsp (Supplementary Table 7). At week 26, the percentage of time spent in target IG range (4.0–10.0 mmol/L [71–180 mg/dL]) was 53% with mealtime and postmeal faster aspart and 51% with mealtime IAsp, whereas time spent in low IG (≤3.9 mmol/L [≤70 mg/dL]) was ∼6.0% in all treatment arms. The mean of the coefficient of variation in the IG profile was similar from baseline to week 26 for all treatments (Supplementary Table 7).
PPG increment results at baseline and 26 weeks after randomization are presented in Supplementary Fig. 6. There was no statistically significant difference between mealtime faster aspart and mealtime IAsp in change from baseline after 26 weeks in 30-min, 1-h, or 2-h PPG increment (Supplementary Fig. 6). A statistically significant difference in favor of mealtime IAsp versus postmeal faster aspart was reported at 30 min (ETD 1.64 mmol/L [95% CI 0.72; 2.56], 29.6 mg/dL [13.1; 46.1]; P = 0.001), 1 h (2.77 mmol/L [1.32; 4.22], 49.9 mg/dL [23.8; 76.0]; P < 0.001), and 2 h (2.59 mmol/L [0.56; 4.62], 46.7 mg/dL [10.1; 83.3]; P = 0.013). Similar results were obtained with regard to changes in PPG (data not shown).
Other Secondary End Points
Mean FPG was relatively stable across the three treatment arms from baseline to week 12 and to week 26; however, a large variation in the results was observed due to participant difficulty in handling the FPG home blood sampling kit. No significant difference was reported for mealtime faster aspart or postmeal faster aspart versus mealtime IAsp in change from baseline to week 26 in FPG (Supplementary Table 7).
After 26 weeks, change from baseline in 1,5-AG was statistically significantly in favor of mealtime faster aspart compared with mealtime IAsp (ETD 0.52 μg/mL [95% CI 0.09; 0.95], P = 0.018). There was no statistically significant difference in 1,5-AG between the postmeal faster aspart and mealtime IAsp arms (Supplementary Table 7).
Safety
Hypoglycemia rates are presented in Table 2. The overall rate of severe or BG-confirmed hypoglycemic episodes was comparable between mealtime faster aspart and mealtime IAsp, and also between postmeal faster aspart and mealtime IAsp (estimated rate ratio for both comparisons 1.11 [95% CI 0.90; 1.37]). There was no statistically significant difference in daytime or nocturnal (2300–0700 h, inclusive) severe or BG-confirmed hypoglycemia with mealtime faster aspart versus mealtime IAsp (estimated rate ratios 1.10 [95% CI 0.89; 1.35] and 1.29 [0.93; 1.79], respectively). Daytime severe or BG-confirmed hypoglycemia was not statistically different between postmeal faster aspart and mealtime IAsp (estimated rate ratio 1.07 [95% CI 0.86; 1.32]), although nocturnal severe or BG-confirmed hypoglycemia was statistically significantly different in favor of mealtime IAsp (estimated rate ratio 1.50 [95% CI 1.09; 2.08], P = 0.014). Meal-related hypoglycemia rates are also shown in Table 2.
Table 2.
Treatment-emergent hypoglycemic events
| Faster aspart (mealtime) |
Faster aspart (postmeal) |
IAsp (mealtime) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | E | R | n | % | E | R | n | % | E | R | |
| Treatment-emergent hypoglycemia | ||||||||||||
| Severe* | 3 | 1.1 | 3 | 0.02 | 8 | 3.1 | 8 | 0.06 | 4 | 1.6 | 4 | 0.03 |
| Severe or BG confirmed | 228 | 87.4 | 3,583 | 27.91 | 227 | 88.0 | 3,594 | 28.15 | 217 | 84.1 | 3,276 | 25.66 |
| Daytime | 226 | 86.6 | 3,187 | 24.82 | 224 | 86.8 | 3,117 | 24.42 | 217 | 84.1 | 2,963 | 23.21 |
| Nocturnal† | 112 | 42.9 | 396 | 3.08 | 125 | 48.4 | 477 | 3.74 | 104 | 40.3 | 313 | 2.45 |
| Meal-related severe or BG-confirmed hypoglycemia | ||||||||||||
| Within 1 h after a meal | 64 | 24.5 | 119 | 0.93 | 46 | 17.8 | 66 | 0.52 | 62 | 24.0 | 105 | 0.82 |
| Within 2 h after a meal | 161 | 61.7 | 717 | 5.58 | 136 | 52.7 | 505 | 3.96 | 147 | 57.0 | 601 | 4.71 |
| Within 4 h after a meal | 200 | 76.6 | 1,777 | 13.84 | 201 | 77.9 | 1,782 | 13.96 | 191 | 74.0 | 1,668 | 13.07 |
Safety analysis set. %, percentage of participants; E, number of events; R, event rate per patient-year of exposure.
Treatment emergent was defined as an event that had onset up to 1 day after the last day of randomized treatment and excluding the events occurring in the run-in period. BG-confirmed hypoglycemia was defined as an episode with a plasma glucose value of <3.1 mmol/L (<56 mg/dL) with or without symptoms consistent with hypoglycemia.
*Severe according to ISPAD 2014.
†Nocturnal severe or BG-confirmed hypoglycemia was statistically significantly different in favor of mealtime IAsp vs. postmeal faster aspart (estimated rate ratio 1.50 [95% CI 1.09; 2.08], P = 0.014).
After 26 weeks of treatment, estimated change in body weight was +2.2, +1.9, and +2.2 kg with mealtime faster aspart, postmeal faster aspart, and mealtime IAsp, respectively, with no statistical differences between treatment arms (Supplementary Table 9). Mean and median daily bolus insulin doses increased as expected in childhood over the study by similar amounts across all treatment arms (Supplementary Table 9), with no major change in the basal/bolus ratio 26 weeks after randomization (Supplementary Table 9).
No clinically relevant differences were observed in the adverse event profiles across treatment groups during the 26-week treatment period (Supplementary Table 10) or with regard to vital signs, antibody measurements, injection site reactions, body measurements (BMI, body weight, and height), physical examination, Tanner staging, and safety laboratory assessments (biochemistry, hematology, lipids, and urinalysis).
Conclusions
The primary objective of this trial was confirmed, demonstrating that mealtime faster aspart was noninferior to mealtime IAsp in terms of change in HbA1c 26 weeks after randomization in children and adolescents with type 1 diabetes. Furthermore, for the same end point, mealtime faster aspart was confirmed to be superior to IAsp, and postmeal faster aspart noninferior to IAsp. Glycemic control, assessed by eight-point SMBG and CGM IG profiles 26 weeks after randomization, demonstrated a significant improvement in 1-h PPG and IG increment over the mean of all meals with mealtime faster aspart compared with IAsp, although this was not demonstrated in a subgroup of patients following a standardized liquid-meal test. Compared with faster aspart administered 20 min after a meal, changes in SMBG and IG increments were found to be generally in favor of mealtime IAsp. The change from baseline in 1,5-AG also significantly favored mealtime faster aspart over IAsp, supporting a reduction in postprandial hyperglycemic excursions with mealtime faster aspart. Over the course of the study, the incidence of overall hypoglycemia was similar between treatments. There were no differences in timing of hypoglycemia (daytime or nocturnal) between mealtime faster aspart and IAsp, although nocturnal hypoglycemia was reported more often with postmeal faster aspart versus IAsp. We observed no major difference in insulin dosing across the three groups.
These findings are in general alignment with similar studies in adults with type 1 diabetes (17,18). When administered as part of a basal-bolus regimen with insulin detemir (onset 1), mealtime faster aspart was associated with a statistically significant improvement in HbA1c with IAsp after 26 weeks of treatment (ETD −0.15% [95% CI −0.23; −0.07], −1.62 mmol/mol [−2.50; −0.73]; P = 0.0003) and a superior reduction in 2-h PPG increment (meal test) (−0.67 mmol/L [−1.29; −0.04], −12.01 mg/dL [−23.33; −0.70]; P = 0.0375) (18). In participants receiving insulin degludec (onset 8), noninferiority was demonstrated for change from baseline in HbA1c with mealtime and postmeal faster aspart versus IAsp, and mealtime faster aspart was superior to IAsp for 1-h PPG increment (meal test) (ETD −0.90 mmol/L [−1.36; −0.45], −16.24 mg/dL [−24.42; −8.05]; P < 0.001) (17). Rates of overall severe or BG-confirmed hypoglycemia were comparable between treatments across both previous trials, although differences in meal-related hypoglycemia were reported (17,18).
The findings reported here with faster aspart in combination with insulin degludec are clinically important given the need for exogenous insulin replacement to mimic physiology as closely as possible in children and adolescents (5). International Society for Pediatric and Adolescent Diabetes (ISPAD) guidelines recommend bolus insulin administration before meals (5), and the current study shows glycemic control to be significantly improved with mealtime faster aspart compared with IAsp. Previous studies suggest that postprandial administration of bolus insulin can be a safe, effective alternative in children and adolescents (19). The current trial shows that postmeal faster aspart is noninferior to IAsp in terms of HbA1c control, with a comparable risk of overall hypoglycemia, which supports user flexibility without compromising overall HbA1c control. However, there were higher postprandial BG levels during the meal test with postmeal faster aspart compared with mealtime IAsp, indicating that flexibility of postmeal administration comes at the cost of less tight postprandial glycemic control and that mealtime dosing is generally preferable with high-carbohydrate meals (such as the liquid meal used in the meal test).
The rate of nocturnal severe or BG-confirmed hypoglycemic episodes was also higher with postmeal faster aspart versus mealtime IAsp. These episodes occurred predominantly from 2200 to 0100 h and 0600 to 0700 h, with no noticeable differences at other times during the night.
This is the first study to evaluate efficacy and safety of an ultra–fast-acting insulin analog in children and adolescents. Strengths of the study include the large patient sample and high retention rate. Insulin degludec was optimized during a 12-week run-in period to enable assessment of the study bolus insulin, and a double-blind design (open-label for postmeal faster aspart) and use of several glycemic measures, including SMBG and CGM, allowed a thorough efficacy assessment.
During the run-in, a reduction in mean HbA1c was observed for participants subsequently randomized to each of the three treatment groups, and HbA1c was close to the treatment target of 7.5% (58 mmol/mol) at baseline. During the treatment period, HbA1c remained stable in the mealtime faster aspart group and increased in the IAsp and postmeal faster aspart groups. The lack of further HbA1c reduction may be partly due to a reluctance to intensively titrate insulin dose in this relatively well-controlled population due to fear of hypoglycemia. A similar initial reduction followed by a flat or increasing HbA1c level has been observed in other pediatric trials (20,21), which may reflect the challenges of diabetes management and insulin therapy in children and adolescents with type 1 diabetes (12,22,23).
Limitations of the study include the use of a home-sampling kit to measure FPG, which participants found challenging and may have led to variation in the results, and the small sample of trial participants for some assessments (liquid-meal test and CGM end points). In addition, the prespecified identical dosing of insulin during the liquid-meal test at week 0 (baseline) and 26 may have obviated the opportunity to identify differences in PPG excursions at week 26. Nonetheless, the CGM IG values are likely more reflective of real-life experiences than the liquid-meal test and demonstrated lower excursions with mealtime faster aspart compared with IAsp.
Conclusion
The continuous improvement in long-term glycemic control, management of hypoglycemia risk, and dosing flexibility with intensified insulin therapy is imperative in children and adolescents. The onset 7 trial demonstrated a significant improvement in change in HbA1c from baseline with mealtime faster aspart versus mealtime IAsp and noninferior HbA1c control with postmeal administration of faster aspart compared with mealtime IAsp in children and adolescents with type 1 diabetes. Furthermore, postprandial hyperglycemia was significantly reduced with mealtime faster aspart versus IAsp, and overall hypoglycemia and safety profiles were comparable across all treatments.
Supplementary Material
Article Information
Acknowledgments. The authors thank all investigators, trial staff, and participants of this study.
Funding and Duality of Interest. The onset 7 trial, including study design, data collection, analysis, and interpretation, was funded by Novo Nordisk A/S. Medical writing and editorial assistance were provided by Samuel Bestall and Erin Slobodian (Watermeadow Medical, an Ashfield company, part of UDG Healthcare PLC), funded by Novo Nordisk A/S. B.W.B. reports grants and personal fees from Abbott, AstraZeneca, BD, Boehringer Ingelheim, Dexcom, Diasome, GlaxoSmithKline, Insulet, Janssen, Lexicon, Eli Lilly and Company, MannKind, Medtronic, Novo Nordisk, Pfizer, and Sanofi and holds shares with Aseko. V.I. has acted as a speaker and/or an advisory board member for Berlin-Chemie, Eli Lilly and Company, Novo Nordisk, Pfizer, Sandoz, Sanofi, and Shire. M.K. has acted as a speaker for Berlin-Chemie, LifeScan, Medtronic, Novo Nordisk, and Sanofi and has received honoraria from Novo Nordisk as a member of the Novo Nordisk Paediatric Type 2 Diabetes Global Expert Panel. L.M.L. has served as a consultant for Johnson & Johnson, Eli Lilly and Company, Sanofi, Novo Nordisk, MannKind, Merck, Bristol-Myers Squibb, AstraZeneca, Roche, Dexcom, Unomedical-ConvaTec, Insulet, and Boehringer Ingelheim and has received research grants from Sanofi, Novo Nordisk, Dexcom, Insulet, and Boehringer Ingelheim. P.V.R. received research grants, clinical trial payments, or personal fees from Novo Nordisk, Sanofi, Eli Lilly and Company, USV Pharmaceuticals, Ipca Laboratories, Hetero Pharma, Panacea Biotec, Torrent Pharma, SMS Pharma, Dr. Reddy’s, Biocon and Clinigene, Aventis and PHRI (Canada), Kowa and Pharmanet, Emisphere and IGate, Bristol-Meyers Squibb and Icon, Merck and Clinigene, Metabolex and Icon, Phenomix and IGate, Biodel and Clinigene, Macrogenics and IGate, ActiveX Biosciences and Medpace, Daiichi Sankyo and Icon, XTL Development and GVK Biosciences, Johnson & Johnson Pharma, Boehringer Ingelheim and SIRO, Novartis Health Care and Academic Alliance for Clinical Trials, Quintiles, and IQVIA and holds shares with DiabetOmics, Inc. S.D., M.E., and S.F.L. are employees of Novo Nordisk. M.E. holds shares with Novo Nordisk. T.D. has acted as consultant, advisory board member, steering committee member, or speaker for Abbott, Medtronic, Roche, Lexicon, Menarini, Boehringer Ingelheim, AstraZeneca, Novo Nordisk, Sanofi, Dexcom, and Eli Lilly and Company and has received research grants from Abbott, AstraZeneca, Novo Nordisk, Medtronic, and Sanofi. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. B.W.B., V.I., M.K., L.M.L., P.V.R., S.D., M.E., S.F.L., and T.D. contributed to data interpretation, reviewed and contributed to the content of the manuscript, had authority in the decision to submit the manuscript, and approved the manuscript for publication. B.W.B. and T.D. were the principal investigators of this clinical trial. M.E. was the medical specialist for the trial and had the medical responsibility on a clinical trial level. S.F.L. was the responsible statistician. B.W.B. and T.D. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Data Availability. The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Prior Presentation. Parts of this study were presented in poster form at the 44th Annual Conference of ISPAD, Hyderabad, India, 11–14 October 2018.
Footnotes
Clinical trial reg. no. NCT02670915, clinicaltrials.gov
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-0009/-/DC1.
This article is featured in a podcast available at http://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.Simmons KM, Michels AW. Type 1 diabetes: a predictable disease. World J Diabetes 2015;6:380–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson CC, Gyürüs E, Rosenbauer J, et al. . Trends in childhood type 1 diabetes incidence in Europe during 1989-2008: evidence of non-uniformity over time in rates of increase. Diabetologia 2012;55:2142–2147 [DOI] [PubMed] [Google Scholar]
- 3.International Diabetes Federation. IDF Diabetes Atlas , 8th edition: global fact sheet [Internet], 2017. Available from http://diabetesatlas.org/resources/2017-atlas.html. Accessed 29 November 2018
- 4.Chiang JL, Maahs DM, Garvey KC, et al. . Type 1 diabetes in children and adolescents: a position statement by the American Diabetes Association. Diabetes Care 2018;41:2026–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danne T, Phillip M, Buckingham BA, et al. . ISPAD Clinical Practice Consensus Guidelines 2018: insulin treatment in children and adolescents with diabetes. Pediatr Diabetes 2018;19(Suppl. 27):115–135 [DOI] [PubMed] [Google Scholar]
- 6.Beckles ZL, Edge JA, Mugglestone MA, Murphy MS, Wales JK; Guideline Development Group . Diagnosis and management of diabetes in children and young people: summary of updated NICE guidance. BMJ 2016;352:i139. [DOI] [PubMed] [Google Scholar]
- 7.National Institute for Health and Care Excellence (NICE). Diabetes (type 1 and type 2) in children and young people: diagnosis and management [Internet], 2015. Available from https://www.nice.org.uk/guidance/ng18/resources/diabetes-type-1-and-type-2-in-children-and-young-people-diagnosis-and-management-1837278149317. Accessed 29 November 2018
- 8.Miller KM, Foster NC, Beck RW, et al.; T1D Exchange Clinic Network . Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care 2015;38:971–978 [DOI] [PubMed] [Google Scholar]
- 9.Donaghue KC, Marcovecchio ML, Wadwa RP, et al. . ISPAD Clinical Practice Consensus Guidelines 2018: microvascular and macrovascular complications in children and adolescents. Pediatr Diabetes 2018;19(Suppl. 27):262–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diabetes Control and Complications Trial Research Group Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr 1994;125:177–188 [DOI] [PubMed] [Google Scholar]
- 11.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 2003;290:2159–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cengiz E. Undeniable need for ultrafast-acting insulin: the pediatric perspective. J Diabetes Sci Technol 2012;6:797–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cemeroglu AP, Kleis L, Wood A, Parkes C, Wood MA, Davis AT. Comparison of the effect of insulin glulisine to insulin aspart on breakfast postprandial blood glucose levels in children with type 1 diabetes mellitus on multiple daily injections. Endocr Pract 2013;19:614–619 [DOI] [PubMed] [Google Scholar]
- 14.Heise T, Pieber TR, Danne T, Erichsen L, Haahr H. A pooled analysis of clinical pharmacology trials investigating the pharmacokinetic and pharmacodynamic characteristics of fast-acting insulin aspart in adults with type 1 diabetes. Clin Pharmacokinet 2017;56:551–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen G, Meiffren G, Lamers D, et al. . Ultra-rapid BioChaperone Lispro improves postprandial blood glucose excursions vs insulin lispro in a 14-day crossover treatment study in people with type 1 diabetes. Diabetes Obes Metab 2018;20:2627–2632 [DOI] [PubMed] [Google Scholar]
- 16.Fath M, Danne T, Biester T, Erichsen L, Kordonouri O, Haahr H. Faster-acting insulin aspart provides faster onset and greater early exposure vs insulin aspart in children and adolescents with type 1 diabetes mellitus. Pediatr Diabetes 2017;18:903–910 [DOI] [PubMed] [Google Scholar]
- 17.Buse JB, Carlson AL, Komatsu M, et al. . Fast-acting insulin aspart versus insulin aspart in the setting of insulin degludec-treated type 1 diabetes: efficacy and safety from a randomized double-blind trial. Diabetes Obes Metab 2018;20:2885–2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell-Jones D, Bode BW, De Block C, et al. . Fast-acting insulin aspart improves glycemic control in basal-bolus treatment for type 1 diabetes: results of a 26-week multicenter, active-controlled, treat-to-target, randomized, parallel-group trial (onset 1). Diabetes Care 2017;40:943–950 [DOI] [PubMed] [Google Scholar]
- 19.Danne T, Aman J, Schober E, et al.; ANA 1200 Study Group . A comparison of postprandial and preprandial administration of insulin aspart in children and adolescents with type 1 diabetes. Diabetes Care 2003;26:2359–2364 [DOI] [PubMed] [Google Scholar]
- 20.Thalange N, Deeb L, Iotova V, et al. . Insulin degludec in combination with bolus insulin aspart is safe and effective in children and adolescents with type 1 diabetes. Pediatr Diabetes 2015;16:164–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thalange N, Bereket A, Larsen J, Hiort LC, Peterkova V. Insulin analogues in children with type 1 diabetes: a 52-week randomized clinical trial. Diabet Med 2013;30:216–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perantie DC, Lim A, Wu J, et al. . Effects of prior hypoglycemia and hyperglycemia on cognition in children with type 1 diabetes mellitus. Pediatr Diabetes 2008;9:87–95 [DOI] [PubMed] [Google Scholar]
- 23.Streisand R, Monaghan M. Young children with type 1 diabetes: challenges, research, and future directions. Curr Diab Rep 2014;14:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.