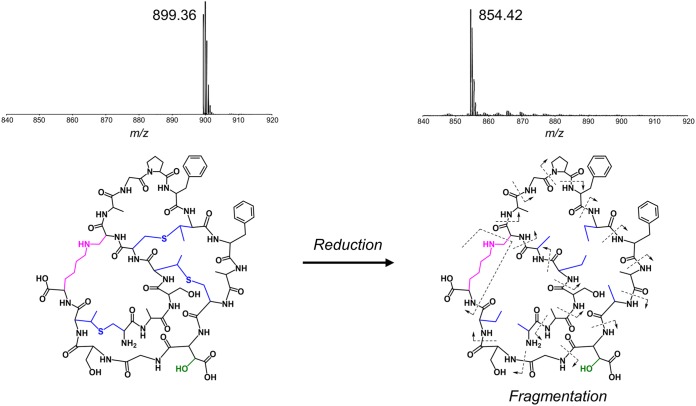
FIG 3.
Characterization of kyamicin. The connectivity of the peptide was confirmed by chemical reduction followed by tandem MS fragmentation. Reduction with NaBH4-NiCl2 resulted in the cleavage of the methyllanthionine bridges (blue), corresponding to the loss of three S atoms and gain of six H atoms, with a mass shift from m/z 899.36 ([M + 2H]2+) to m/z 854.42 ([M + 2H]2+). Tandem MS using the MALDI-TOF LIFT method allowed identification of the y ion (NH3+) series for the complete peptide (see Fig. S5 in the supplemental material). Fragmentation of the lysinoalanine bridge (pink) occurred via rearrangement to give N=CH2 at the terminus of the lysine side chain and a glycine residue at position 6.