Figure 1.
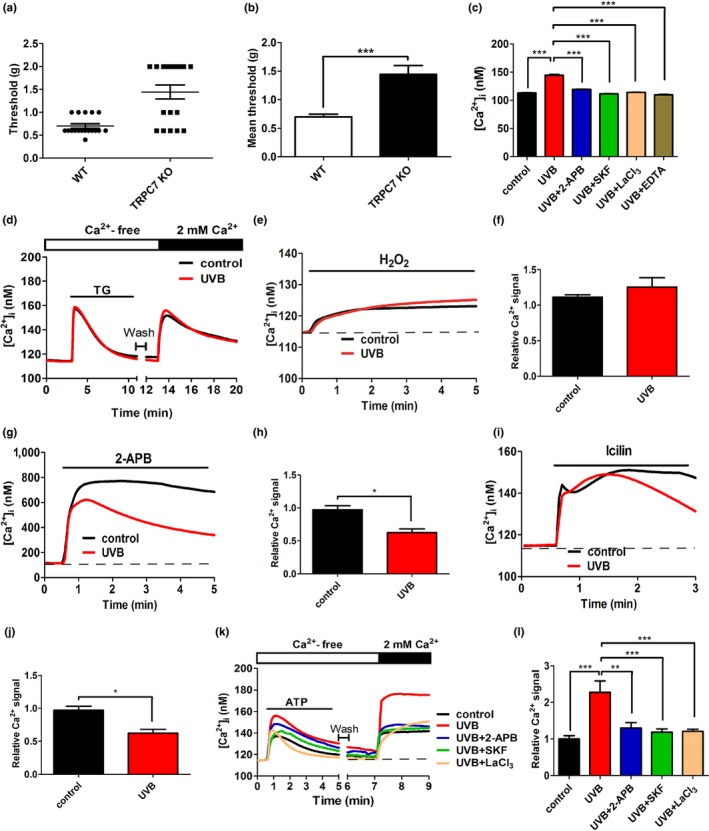
Physiologic role of TRPC7 as a nociceptive mechanoreceptor and the specificity of its cellular mechanism to the TRPC family. (a) Calibrated von Frey filaments were used to assess mechanical hyperalgesia in wild‐type (WT, n = 5) and TRPC7−/− knockout mice (KO, n = 4). The threshold grams of force that elicited a response in WT and TRPC7−/− knockout mice were recorded and averaged. (b) The mean (± SD) thresholds were significantly different between wild‐type and TRPC7−/− knockout mice. (c) TRPC7 initiated UVB‐induced [Ca2+]i elevation in human primary keratinocytes. The mean (± SD) [Ca2+]i from 195 keratinocytes with or without UVB irradiation after a 30‐min pretreatment with 1 mM EDTA (extracellular Ca2+ chelator) or a TRPC inhibitor (50 μM 2‐APB, 25 μM SKF96365 [SKF], or 100 μM LaCl3). (d) UVB irradiation did not affect thapsigargin (TG)‐induced store‐operated Ca2+ mobilization or Ca2+ store depletion‐induced Ca2+ influx in keratinocytes. Intracellular Ca2+ responses were measured by using Ca2+ imaging after treating keratinocytes with (e) hydrogen peroxide (H2O2; mean ± SD, n = 285 cells [f]), (g) 2‐aminoethoxydiphenyl borate (2‐APB; mean ± SD, n = 285 cells [h]), or (i) icilin (mean ± SD, n = 285 cells [j]). (k) After the application of ATP to activate TRPCs via the phospholipase C (PLC) pathway in Ca2+‐free balanced salt solution, extracellular CaCl2 was added. (l) The mean (± SD) area under the [Ca2+]i response curves from 250 keratinocytes after the addition of extracellular CaCl2. The duration of application is indicated by thin black bars. Student's t test: *p < .05, **p < .01, ***p < .001
