Figure 3.
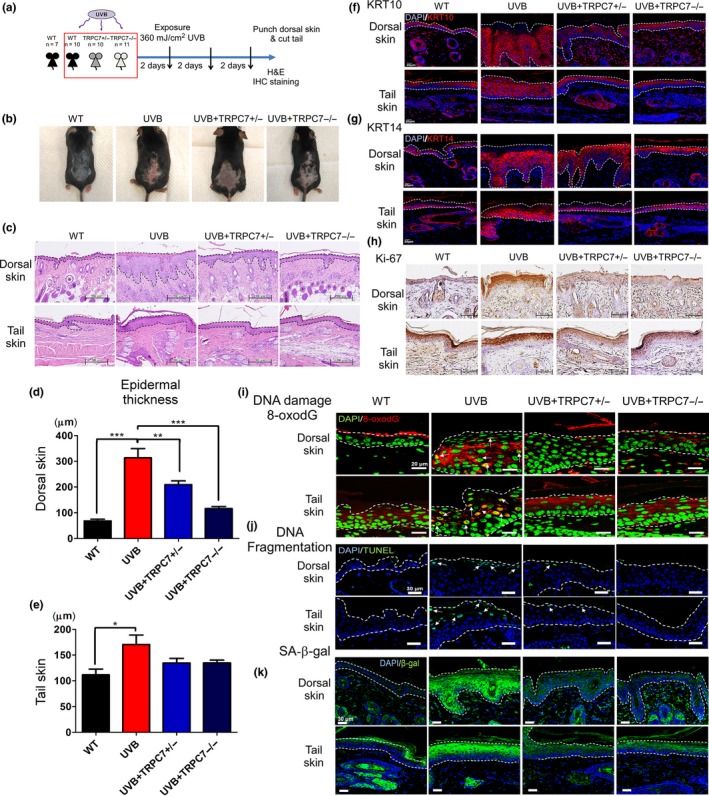
Role of ultraviolet B (UVB)‐induced [Ca2+]i elevation via TRPC7 in initiating epidermal aging, abnormal differentiation, and cell senescence through oxidative stress and DNA damage response activation. (a) A schematic showing the experimental design (wild‐type [WT], n = 7; UVB, n = 10; TRPC7+/−, n = 10 and TRPC7−/−, n = 11). Mice were exposed to UVB three times weekly. Dorsal skin and tail skin punches or clippings were sectioned for histologic and immunohistochemical analysis. (b) Photographs showing the skin's appearance. (c) Hematoxylin‐and‐eosin–stained images of dorsal skin and tail skin. Dotted lines indicate the boundaries of the epidermis. Quantification of the mean (± SD) epidermal thickness in (d) dorsal skin and (e) tail skin. *p < .05; **p < .01; ***p < .001. Differentiating cells were detected by using (f) keratin 10 (KRT10; keratinocytes) and (g) keratin 14 (KRT14; epidermis). (h) Ki‐67 expression is shown, indicating proliferating epidermal cells (brown, counterstained with hematoxylin). Activation of the DNA damage response was analyzed by using (i) 8‐oxo‐2′‐deoxyguanosine (8‐oxodG) staining and (j) terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining. White arrows indicate cells with DNA damage. (k) Cell senescence was determined by detecting senescence‐associated β‐galactosidase (SA‐β‐gal) activity. SA‐β‐gal activity was reduced in the skin of TRPC7 knockout mice
