Fig. 1.
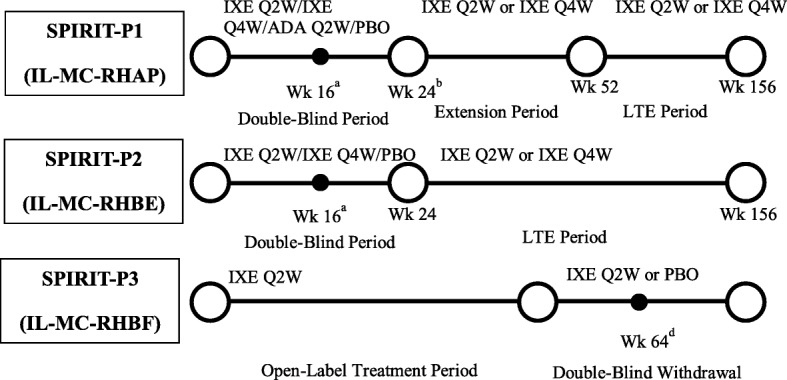
Study design. All patients treated with IXE had a loading dose of 160 mg at week 0. ADA dose was 40 mg Q2W unless stated otherwise. aPatients determined to be inadequate responders by blinded criteria given adjustments to their background/existing therapy. Inadequate responders in the non-IXE groups randomized to IXE Q2W or IXE Q4W with washout for ADA inadequate responders. bResponders in ADA or PBO groups re-randomized to either IXE Q2W or IXE Q4W. cPatients randomized to IXE Q2W or PBO if they met the randomized withdrawal (RW) criteria (i.e., those who met Coates criteria for MDA for ≥ 3 consecutive months across ≥ 4 consecutive visits) at week 36 or later up to week 64. dPatients who had not met RW criteria at week 64 were given IXE Q2W; patients who relapsed (no longer met MDA criteria) during the double-blind withdrawal period were switched to, or continued, IXE Q2W. ADA Q2W: 40 mg of adalimumab every 2 weeks; IXE Q2W: 80 mg of ixekizumab every 2 weeks; IXE Q4W: 80 mg of ixekizumab every 4 weeks; LTE: long-term extension; MDA: minimal disease activity; PBO: placebo; Wk: week
