Abstract
CD133, also known as prominin-1, was first described as a cell surface marker on early progenitor and hematopoietic stem cells. It is a five-domain transmembrane protein composed of an N-terminal extracellular tail, two small cytoplasmic loops, two large extracellular loops containing seven potential glycosylation sites, and a short C-terminal intracellular tail. CD133 has been used as a marker to identify cancer stem cells derived from primary solid tumors and as a prognostic marker of gliomas. Herein, we developed novel anti-CD133 monoclonal antibodies (mAbs) and characterized their efficacy in flow cytometry, Western blot, and immunohistochemical analyses. We expressed the full length of CD133 in LN229 glioblastoma cells, immunized mice with LN229/CD133 cells, and performed the first screening using flow cytometry. After limiting dilution, we established 100 anti-CD133 mAbs, reacting with LN229/CD133 cells but not with LN229 cells. Subsequently, we performed the second and third screening with Western blot and immunohistochemical analyses, respectively. Among 100 mAbs, 11 strongly reacted with CD133 in Western blot analysis. One of 11 clones, CMab-43 (IgG2a, kappa), showed a sensitive and specific reaction against colon cancer cells, warranting the use of CMab-43 in detecting CD133 in pathological analyses of CD133-expressing cancers.
Keywords: : CD133, monoclonal antibody, immunohistochemistry, colon cancer
Introduction
Cancer stem cells (CSCs) share many of the properties of non-neoplastic stem cells. They are also characterized by extensive proliferation, self-renewal, invasion, metastasis, and drug resistance.(1–3) Side-population cells and several protein markers specific to CSCs have been developed to isolate CSCs from cancer tissues and to investigate the CSC properties in cancer tissues.(4) These CSC markers include CD133 and CD44.(1,5–12)
CD133, also known as prominin-1, was first described as a cell surface marker on hematopoietic stem cells.(13) It is a five-transmembrane glycoprotein composed of an N-terminal extracellular tail, two small cytoplasmic loops, two large extracellular loops containing several potential glycosylation sites, and a short C-terminal intracellular tail.(14) CD133 has been used as a marker to identify CSCs derived from primary solid tumors.(1) Its expression is also used as a prognostic marker of gliomas.(15)
Herein, we produced sensitive and specific monoclonal antibodies (mAbs) against CD133, which can be used for flow cytometry, Western blot, and immunohistochemical analysis.
Materials and Methods
Cell lines
LN229, HCT-116, Chinese hamster ovary (CHO)-K1, Caco-2, and P3X63Ag8U.1 (P3U1) cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA). LN229/CD133 and CHO/CD133 were produced by transfecting pCAG/PA-CD133-RAP-MAP into LN229 and CHO-K1 cells using Neon transfection system (Thermo Fisher Scientific, Inc., Waltham, MA) and Lipofectamine LTX (Thermo Fisher Scientific, Inc.), respectively. A few days after transfection, PA tag-positive cells(16) were sorted using a cell sorter (SH800; Sony Corp., Tokyo, Japan). CHO-K1, CHO/CD133, and P3U1 cell lines were cultured in RPMI 1640 medium (Nacalai Tesque, Inc., Kyoto, Japan), and LN229, LN229/CD133, HCT-116, and Caco-2 cell lines were cultured in Dulbecco's modified Eagle's medium (DMEM) (Nacalai Tesque, Inc.), supplemented with 10% heat-inactivated fetal bovine serum (Thermo Fisher Scientific, Inc.), 100 units/mL of penicillin, 100 μg/mL of streptomycin, and 25 μg/mL of amphotericin B (Nacalai Tesque, Inc.) at 37°C in a humidified atmosphere containing 5% CO2 and 95% air.
Production of hybridoma
Female 4-week-old BALB/c mice were purchased from CLEA Japan (Tokyo, Japan). Animals were housed under specific pathogen-free conditions. The Animal Care and Use Committee of Tohoku University approved all of the animal experiments described herein.
Mice were immunized using intraperitoneal (i.p.) injections of LN229/CD133 cells together with Imject Alum (Thermo Fisher Scientific, Inc.). After several additional immunizations, a booster injection of LN229/CD133 cells was intraperitoneally administered 2 days before harvesting spleen cells. Spleen cells were then fused with P3U1 cells using PEG1500 (Roche Diagnostics, Indianapolis, IN) or GenomONE-CF (Ishihara Sangyo Kaisha, Ltd., Osaka, Japan). The resulting hybridomas were grown in RPMI medium supplemented with hypoxanthine, aminopterin, and thymidine selection medium supplement (Thermo Fisher Scientific, Inc.).
Culture supernatants were screened using flow cytometry (first screening), Western blot (second screening), and immunohistochemical analyses (third screening). MAbs were purified from supernatants of hybridomas cultured in Hybridoma-SFM medium (Thermo Fisher Scientific, Inc.) using Protein G Sepharose 4 Fast Flow (GE Healthcare United Kingdom, Ltd., Buckinghamshire, England).
Flow cytometry
Cells were harvested by brief exposure to 0.25% trypsin/1 mM ethylenediaminetetraacetic acid (EDTA) (Nacalai Tesque, Inc.). After washing with 0.1% bovine serum albumin (BSA)/phosphate buffered saline (PBS), the cells were treated with 1 μg/mL of anti-CD133 mAb (clone CMab-43) for 30 min at 4°C and then with Alexa Fluor 488-conjugated antimouse IgG (1:1000; Cell Signaling Technology, Inc., Danvers, MA). Fluorescence data were collected using EC800 or SA3800 Cell Analyzers (Sony Corp.).
Western blot analysis
Cell lysates (10 μg) were boiled in sodium dodecyl sulfate sample buffer (Nacalai Tesque, Inc.) and proteins were then electrophoresed on 5%–20% polyacrylamide gels (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and were transferred onto polyvinylidene difluoride (PVDF) membranes (Merck KGaA, Darmstadt, Germany). After blocking with 4% skim milk (Nacalai Tesque, Inc.), membranes were incubated with 1 μg/mL of anti-CD133 mAb (clone CMab-43) and anti-β-actin (clone AC-15; Sigma-Aldrich Corp., St. Louis, MO), and then with peroxidase-conjugated antimouse IgG (1:1000 diluted; Agilent Technologies, Inc., Santa Clara, CA), and were finally developed using ImmunoStar LD (Wako Pure Chemical Industries, Ltd.) using a Sayaca-Imager (DRC Co., Ltd., Tokyo, Japan).
Determination of binding affinity using flow cytometry
LN229/CD133 and Caco-2 cells (2 × 105) were suspended in 100 μL of serially diluted mAbs (6–100 μg/mL), and Alexa Fluor 488-conjugated antimouse IgG (1:200; Cell Signaling Technology, Inc.) was then added. Fluorescence data were collected using a cell analyzer (EC800; Sony Corp.). The dissociation constants (KD) were calculated by fitting the binding isotherms using the built-in one-site binding models in GraphPad PRISM 6 (GraphPad software, Inc., La Jolla, CA).
Immunohistochemical analyses
Colon cancer tissues were purchased from U.S. Biomax, Inc. (Rockville, MD). Histological sections of 4-μm thickness were deparaffinized in xylene and were then rehydrated and autoclaved in citrate buffer (pH 6.0; Agilent Technologies, Inc.) for 20 minutes. Sections were then incubated with 1 μg/mL of CMab-43 for 1 hour at room temperature and were then treated using an Envision+ kit (Agilent Technologies, Inc.) for 30 minutes. Color was developed using 3,3-diaminobenzidine tetrahydrochloride (Agilent Technologies, Inc.) for 2 minutes, and sections were then counterstained with hematoxylin (Wako Pure Chemical Industries, Ltd.).
Results
In this study, we immunized mice with LN229/CD133 cells followed by a booster i.p. injection of LN229/CD133 cells. Culture supernatants were screened using flow cytometry to assess reactions with LN229 and LN229/CD133 cells. A total of 100 clones were generated after limiting dilution. Subsequently, mAbs were selected according to their signal efficacy on Western blot analysis. These analyses identified 79 of the 100 clones that were able to detect CD133, of which 11 exerted a strong and specific reaction. The 11 clones selected by Western blot analysis were further tested, and immunohistochemical analysis demonstrated that 4 clones showed strong staining, 4 showed moderate staining, and 3 showed no staining against colon cancer tissues. Three of the four clones that showed a strong staining against colon cancer tissues were determined to be of the IgG1 subclass; the fourth clone, CMab-43, was of the IgG2a subclass and was selected for the subsequent study.
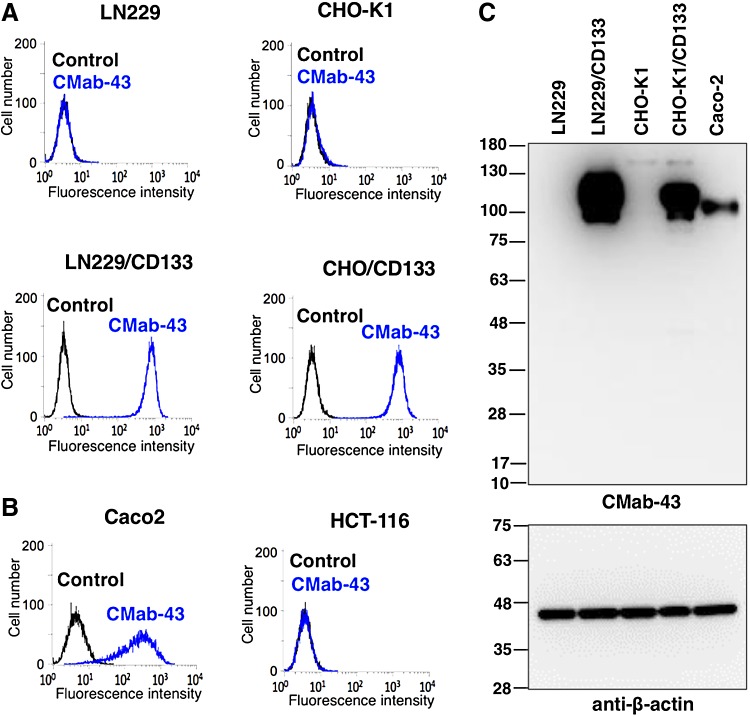
Flow cytometry demonstrated that CMab-43 reacts with LN229/CD133 cells but not with LN229 brain tumor cells (Fig. 1A). CMab-43 reacted with CHO/CD133 cells but not with the CHO-K1 parental cells, indicating that CMab-43 is specific for CD133 (Fig. 1A). CMab-43 also recognized endogenous CD133 in Caco-2 colon cancer cells, but did not react with HCT-116 colon cancer cells (Fig. 1B). On Western blot analyses against LN229, LN229/CD133, CHO-K1, CHO/CD133, and Caco-2 cells, CMab-43 detected a strong signal at ∼100 kDa in LN229/CD133 and CHO/CD133, and did a moderate signal in Caco-2 cells, indicating that CMab-43 is very useful in Western blot analyses (Fig. 1C). The broad band revealed by CMab-43 is likely due to various glycosylation forms of CD133, known to be highly glycosylated.(17)
FIG. 1.
Characterization of CMab-43. (A, B) Flow cytometry with CMab-43; cells were treated with 1 μg/mL of CMab-43 followed by Alexa Fluor 488-conjugated antimouse IgG; black line, negative control. (C) Western blot using CMab-43; cell lysates (10 μg) were electrophoresed and proteins were transferred onto polyvinylidene difluoride (PVDF) membranes. After blocking, membranes were incubated with 1 μg/mL of CMab-43 or anti-β-actin (AC-15) and then incubated with peroxidase-conjugated antimouse IgG.
We further determined the binding affinity of CMab-43 for Caco-2 and LN229/CD133 cells using flow cytometry (Fig. 2). The calculated KD values for CMab-43 against LN229/CD133 and Caco-2 cells are 4.4 × 10−9 M and 2.6 × 10−9 M, respectively, indicating a high affinity for CD133-expressing cell lines.
FIG. 2.
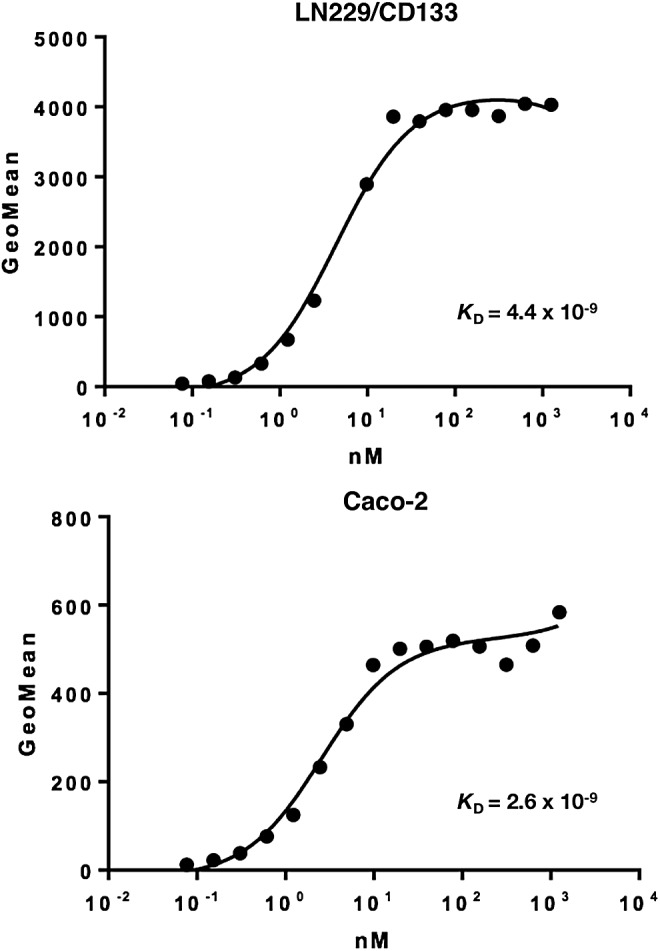
Binding affinity of CMab-43 was determined using flow cytometry. LN229/CD133 and Caco-2 cells were suspended in 100 μL of serially diluted CMab-43 (6 ng/mL–100 μg/mL), and secondary antimouse IgG was then added. Fluorescence data were collected using a cell analyzer.
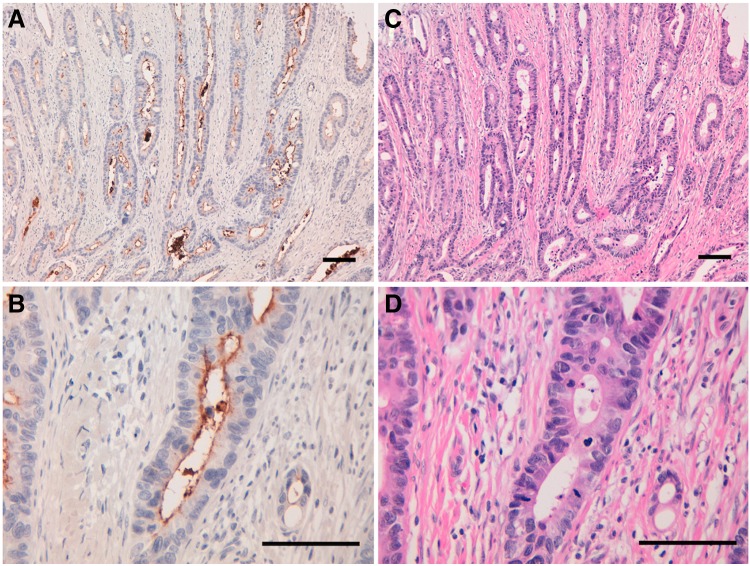
We investigated the immunohistochemical utility of CMab-43 in human colon cancers as high CD133 expression was observed in Caco-2 colon cancer cell lines (Fig. 1C). Recent studies have shown CD133 expression with luminal staining.(18–20) Staining of intraglandular debris was also observed in colorectal cancers. As shown in Figure 3 and Supplementary Figure 1, CMab-43 showed a luminal membrane expression pattern, and intraglandular debris was also stained. Normal mucosae were not positive for CMab-43 staining (Supplementary Fig. S2). CMab-43 stained 5/6 cases (83.3%) of colon adenocarcinomas (Supplementary Fig. S2 and Supplementary Table S1), indicating that CMab-43 is useful for immunohistochemical analysis.
FIG. 3.
Immunohistochemical analysis by CMab-43 against colon cancer tissues. (A, B) Sections were incubated with 1 μg/mL of CMab-43 for 1 hour at room temperature followed by treatment with Envision+ kit for 30 minutes. Color was developed using 3,3-diaminobenzidine tetrahydrochloride for 2 minutes, and sections were then counterstained with hematoxylin. (C, D) Hematoxylin & eosin staining; scale bar = 100 μm.
Discussion
Recently, we produced antipodoplanin (PDPN) cancer-specific mAbs clone LpMab-2(21,22) and LpMab-23,(23,24) which specifically recognize cancer-type PDPN in tumor tissues. For this technology, it is critical that immunogens are produced using cancer cell lines, such as LN229 glioblastoma cells, which express cancer-specific glycan-attached membrane proteins. This method can be used to develop useful mAbs against multiple membrane proteins. Using the same method, we successfully developed clone CMab-43 of IgG2a subclass, which is very useful for Western blot, flow cytometry, and immunohistochemical analysis against CD133. Mouse IgG2a subclass possesses several advantages over mouse IgG1 subclass, including antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity, and IgG2a is also easy to purify using Protein-A or Protein-G.(25,26)
Using LN229/CD133 cells for immunization and first screening, purification of recombinant CD133 proteins is not required. This method may be applicable for developing mAbs, especially against membrane proteins that cannot be easily purified. CMab-43 was highly efficacious in Western blot analyses and produced strong staining in colon cancer cells in immunohistochemical analysis. Hence, CMab-43 will likely be an advantageous tool for the pathological identification of CD133 in many cancers.
Supplementary Material
Acknowledgments
We thank Yoshimi Nakamura for excellent technical assistance. This work was supported, in part, by the Basic Science and Platform Technology Program for Innovative Biological Medicine from Japan Agency for Medical Research and development, AMED (Y.K.). This work was also supported, in part, by the Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research [BINDS]) from AMED (Y.K.), by project for utilizing glycans in the development of innovative drug discovery technologies from AMED (Y.K.), by the Platform for Drug Discovery, Informatics, and Structural Life Science (PDIS) from AMED (Y.K.), and by JSPS KAKENHI Grant Numbers 17K07299 (M.K.K.) and 16K10748 (Y.K.). This work was performed, in part, under the Cooperative Research Program of Institute for Protein Research, Osaka University, CR-17-05, and by the Grant for Joint Research Project of the Institute of Medical Science, the University of Tokyo. The authors thank Enago (www.enago.jp) for the English language review.
Author Disclosure Statement
Y. K. received research funding from Ono Pharmaceutical Co., Ltd. All other authors have nothing to disclose.
References
- 1. Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, and Dirks PB: Identification of human brain tumour initiating cells. Nature 2004;432:396–401 [DOI] [PubMed] [Google Scholar]
- 2. Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U, and Beier CP: CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res 2007;67:4010–4015 [DOI] [PubMed] [Google Scholar]
- 3. Ma S, Lee TK, Zheng BJ, Chan KW, and Guan XY: CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene 2008;27:1749–1758 [DOI] [PubMed] [Google Scholar]
- 4. Nishii T, Yashiro M, Shinto O, Sawada T, Ohira M, and Hirakawa K: Cancer stem cell-like SP cells have a high adhesion ability to the peritoneum in gastric carcinoma. Cancer Sci 2009;100:1397–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rappa G, Fodstad O, and Lorico A: The stem cell-associated antigen CD133 (Prominin-1) is a molecular therapeutic target for metastatic melanoma. Stem Cells 2008;26:3008–3017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, Gordon SA, Shimada Y, and Wang TC: Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells 2009;27:1006–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kai K, Nagano O, Sugihara E, Arima Y, Sampetrean O, Ishimoto T, Nakanishi M, Ueno NT, Iwase H, and Saya H: Maintenance of HCT116 colon cancer cell line conforms to a stochastic model but not a cancer stem cell model. Cancer Sci 2009;100:2275–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, and Clarke MF: Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A 2007;104:10158–10163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Palapattu GS, Wu C, Silvers CR, Martin HB, Williams K, Salamone L, Bushnell T, Huang LS, Yang Q, and Huang J: Selective expression of CD44, a putative prostate cancer stem cell marker, in neuroendocrine tumor cells of human prostate cancer. Prostate 2009;69:787–798 [DOI] [PubMed] [Google Scholar]
- 10. Dembinski JL, and Krauss S: Characterization and functional analysis of a slow cycling stem cell-like subpopulation in pancreas adenocarcinoma. Clin Exp Metastasis 2009;26:611–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shimada Y, Ishii G, Nagai K, Atsumi N, Fujii S, Yamada A, Yamane Y, Hishida T, Nishimura M, Yoshida J, Ikeda N, and Ochiai A: Expression of podoplanin, CD44, and p63 in squamous cell carcinoma of the lung. Cancer Sci 2009;100:2054–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu Z, Hao X, Yan M, Yao M, Ge C, Gu J, and Li J: Cancer stem/progenitor cells are highly enriched in CD133+CD44+ population in hepatocellular carcinoma. Int J Cancer 2010;126:2067–2078 [DOI] [PubMed] [Google Scholar]
- 13. Kobari L, Giarratana MC, Pflumio F, Izac B, Coulombel L, and Douay L: CD133+ cell selection is an alternative to CD34+ cell selection for ex vivo expansion of hematopoietic stem cells. J Hematother Stem Cell Res 2001;10:273–281 [DOI] [PubMed] [Google Scholar]
- 14. Yu Y, Flint A, Dvorin EL, and Bischoff J: AC133-2, a novel isoform of human AC133 stem cell antigen. J Biol Chem 2002;277:20711–20716 [DOI] [PubMed] [Google Scholar]
- 15. Beier D, Wischhusen J, Dietmaier W, Hau P, Proescholdt M, Brawanski A, Bogdahn U, and Beier CP: CD133 expression and cancer stem cells predict prognosis in high-grade oligodendroglial tumors. Brain Pathol 2008;18:370–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fujii Y, Kaneko M, Neyazaki M, Nogi T, Kato Y, and Takagi J: PA tag: A versatile protein tagging system using a super high affinity antibody against a dodecapeptide derived from human podoplanin. Protein Expr Purif 2014;95:240–247 [DOI] [PubMed] [Google Scholar]
- 17. Wang D, Guo Y, Li Y, Li W, Zheng X, Xia H, and Mao Q: Detection of CD133 expression in U87 glioblastoma cells using a novel anti-CD133 monoclonal antibody. Oncol Lett 2015;9:2603–2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fathi A, Mosaad H, Hussein S, Roshdy M, and Ismail EI: Prognostic significance of CD133 and ezrin expression in colorectal carcinoma. IUBMB Life 2017;69:328–340 [DOI] [PubMed] [Google Scholar]
- 19. Kazama S, Kishikawa J, Kiyomatsu T, Kawai K, Nozawa H, Ishihara S, and Watanabe T: Expression of the stem cell marker CD133 is related to tumor development in colorectal carcinogenesis. Asian J Surg 2017. DOI: 10.1016/j.asjsur.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 20. Li W, Lee MR, Choi E, and Cho MY: Clinicopathologic significance of survivin expression in relation to CD133 expression in surgically resected stage II or III colorectal cancer. J Pathol Transl Med 2017;51:17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kato Y, and Kaneko MK: A cancer-specific monoclonal antibody recognizes the aberrantly glycosylated podoplanin. Sci Rep 2014;4:5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaneko MK, Yamada S, Nakamura T, Abe S, Nishioka Y, Kunita A, Fukayama M, Fujii Y, Ogasawara S, and Kato Y: Antitumor activity of chLpMab-2, a human-mouse chimeric cancer-specific antihuman podoplanin antibody, via antibody-dependent cellular cytotoxicity. Cancer Med 2017;6:768–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yamada S, Ogasawara S, Kaneko MK, and Kato Y: LpMab-23: A cancer-specific monoclonal antibody against human podoplanin. Monoclon Antib Immunodiagn Immunother 2017;36:72–76 [DOI] [PubMed] [Google Scholar]
- 24. Kaneko MK, Nakamura T, Kunita A, Fukayama M, Abe S, Nishioka Y, Yamada S, Yanaka M, Saidoh N, Yoshida K, Fujii Y, Ogasawara S, and Kato Y: ChLpMab-23: Cancer-specific human–mouse chimeric anti-podoplanin antibody exhibits antitumor activity via antibody-dependent cellular cytotoxicity. Monoclon Antib Immunodiagn Immunother 2017;36:104–112 [DOI] [PubMed] [Google Scholar]
- 25. Kaneko MK, Nakamura T, Honma R, Ogasawara S, Fujii Y, Abe S, Takagi M, Harada H, Suzuki H, Nishioka Y, and Kato Y: Development and characterization of anti-glycopeptide monoclonal antibodies against human podoplanin, using glycan-deficient cell lines generated by CRISPR/Cas9 and TALEN. Cancer Med 2017;6:382–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kato Y, Kunita A, Fukayama M, Abe S, Nishioka Y, Uchida H, Tahara H, Yamada S, Yanaka M, Nakamura T, Saidoh N, Yoshida K, Fujii Y, Honma R, Takagi M, Ogasawara S, Murata T, and Kaneko MK: Antiglycopeptide mouse monoclonal antibody LpMab-21 exerts antitumor activity against human podoplanin through antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity. Monoclon Antib Immunodiagn Immunother 2017;36:20–24 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.