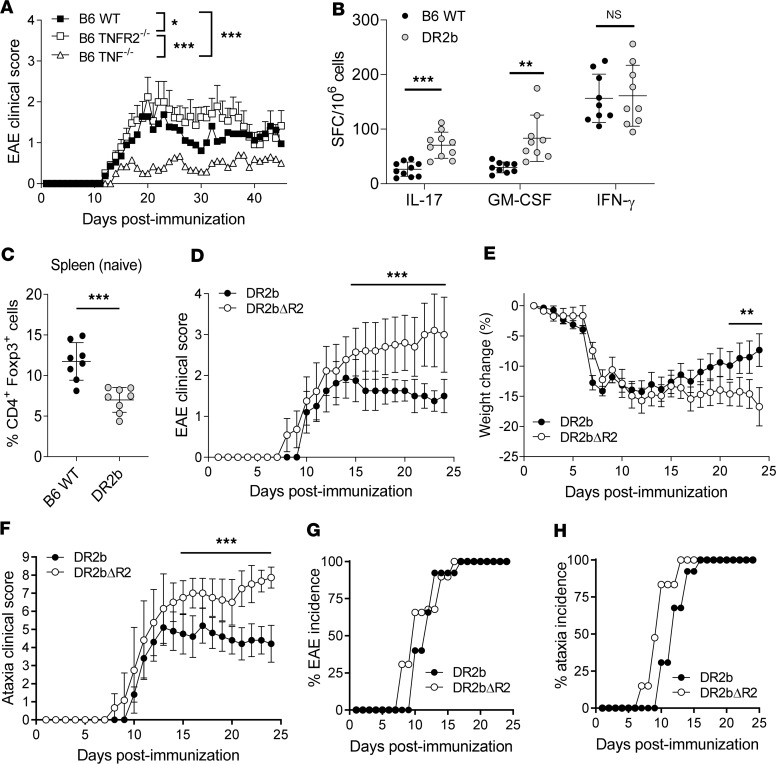
Figure 1. Lack of TNFR2 expression promotes the development of progressive experimental autoimmune encephalomyelitis in HLA-DR2b–transgenic mice.
(A) C57BL/6 (B6) wild-type (WT), B6 TNFR2–/–, and B6 TNF–/– mice were immunized to induce experimental autoimmune encephalomyelitis (EAE). Clinical signs of EAE were monitored daily. Shown are mean clinical disease scores of representative results from 3–6 independent experiments with n = 5–12 mice per group. Statistical significance was determined by 1-way ANOVA corrected for FDR using Benjamini, Krieger, and Yekutieli method. (B) B6 WT and DR2b-transgenic mice were immunized with MOG35-55 peptide in CFA, and the frequencies of Ag-reactive IL-17–, GM-CSF–, and IFN-γ–producing T cells were measured in lymph nodes and spleens at day 9 after immunization. Pooled data from 3 independent experiments, n = 9–10 mice per group. (C) Percentage of CD4+Foxp3+ cells in naive WT B6 mice and naive DR2b mice. Pooled data from 2 independent experiments, n = 8 mice per group. Student’s 2-tailed t test with Welch’s correction. (D–H) DR2b (DR2b+/+ I-A–/–) and DR2bΔR2 (DR2b+/+ I-A–/– TNFR2–/–) were immunized to induce EAE. Shown are representative results from 3–6 independent experiments with n = 5–10 mice per group. (D) Clinical signs of EAE, (E) clinical signs of weight loss, and (F) clinical signs of ataxia were monitored daily. (G) EAE disease incidence and (H) clinical ataxia incidence were evaluated daily. Statistical significance was determined by multiple comparisons with Holm-Šídák correction (B, D–F). NS, not significant; *P ≤ 0.05; **P ≤ 0.01; and ***P ≤ 0.001. Error bars indicate mean ± standard deviation (SD).