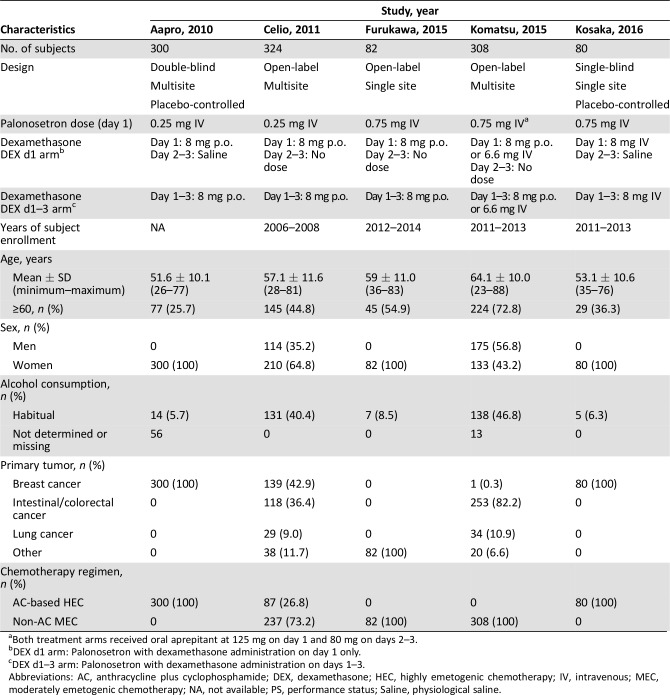
Table 1. Eligible study characteristics and patient demographics.
Both treatment arms received oral aprepitant at 125 mg on day 1 and 80 mg on days 2–3.
DEX d1 arm: Palonosetron with dexamethasone administration on day 1 only.
DEX d1–3 arm: Palonosetron with dexamethasone administration on days 1–3.
Abbreviations: AC, anthracycline plus cyclophosphamide; DEX, dexamethasone; HEC, highly emetogenic chemotherapy; IV, intravenous; MEC, moderately emetogenic chemotherapy; NA, not available; PS, performance status; Saline, physiological saline.