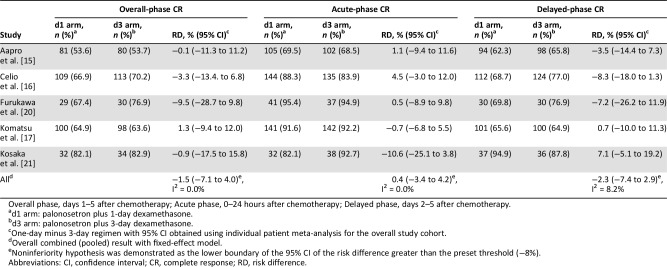
Table 2. Risk differences for CR rates in each study period.
Overall phase, days 1–5 after chemotherapy; Acute phase, 0–24 hours after chemotherapy; Delayed phase, days 2–5 after chemotherapy.
d1 arm: palonosetron plus 1‐day dexamethasone.
d3 arm: palonosetron plus 3‐day dexamethasone.
One‐day minus 3‐day regimen with 95% CI obtained using individual patient meta‐analysis for the overall study cohort.
Overall combined (pooled) result with fixed‐effect model.
Noninferiority hypothesis was demonstrated as the lower boundary of the 95% CI of the risk difference greater than the preset threshold (−8%).
Abbreviations: CI, confidence interval; CR, complete response; RD, risk difference.