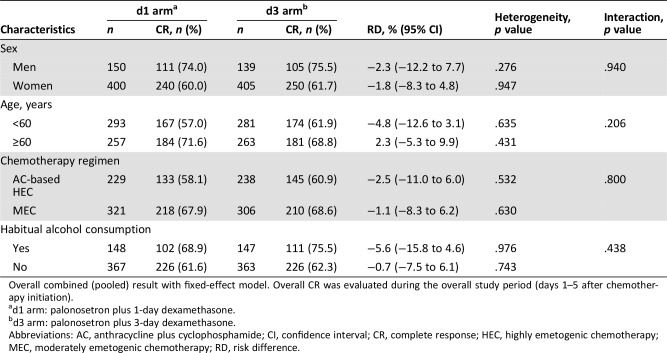
Table 4. Patient characteristic subgroup analysis and interaction for overall CR.
Overall combined (pooled) result with fixed‐effect model. Overall CR was evaluated during the overall study period (days 1–5 after chemotherapy initiation).
d1 arm: palonosetron plus 1‐day dexamethasone.
d3 arm: palonosetron plus 3‐day dexamethasone.
Abbreviations: AC, anthracycline plus cyclophosphamide; CI, confidence interval; CR, complete response; HEC, highly emetogenic chemotherapy; MEC, moderately emetogenic chemotherapy; RD, risk difference.