Table 3. Hematologic AEsa associated with permanent or temporary treatment discontinuations and dose reductions.
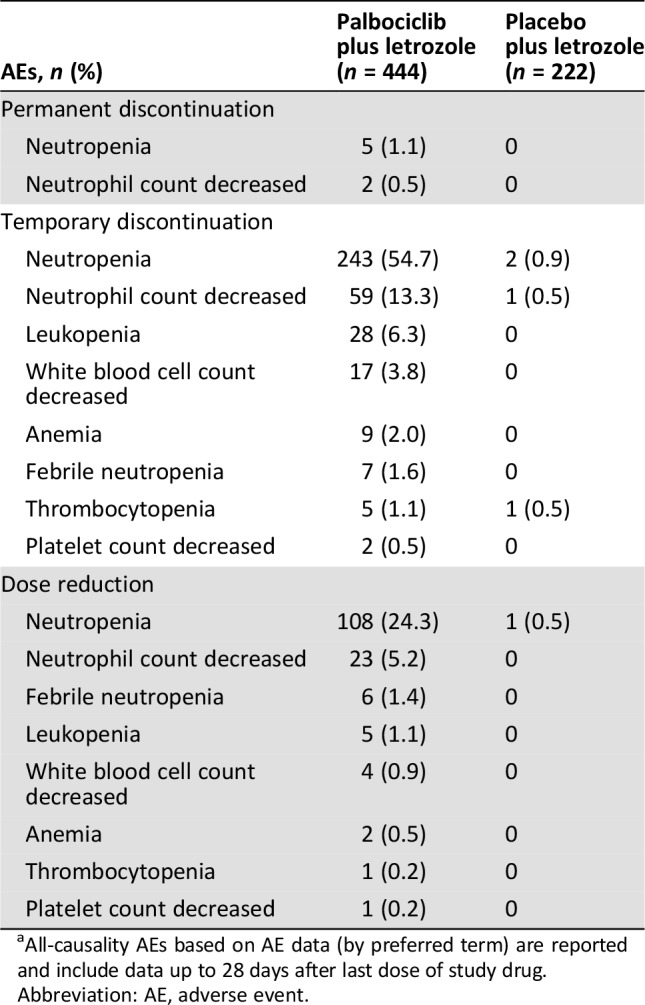
All‐causality AEs based on AE data (by preferred term) are reported and include data up to 28 days after last dose of study drug.
Abbreviation: AE, adverse event.
