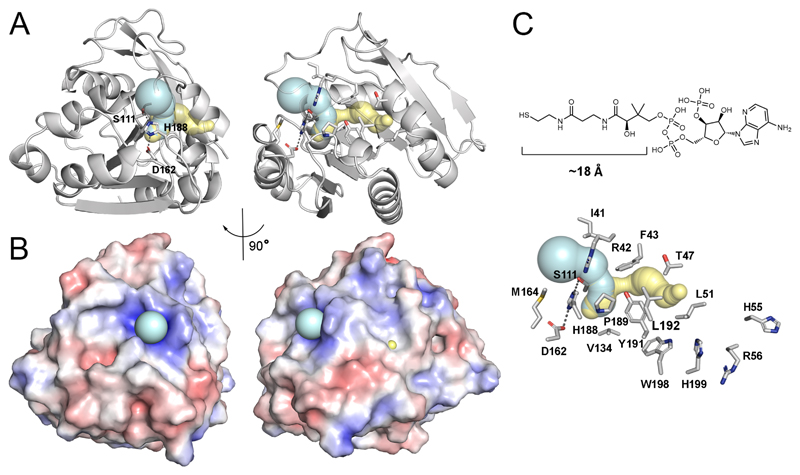
Figure 8. A putative active site tunnel in human ABHD14B.
(A) The tunnels calculated by CAVER (teal) and MOLEonline (yellow) are essentially identical albeit slightly different in length using the available structure of human ABHD14B (PDB: 1IMJ)23. The entrance of the tunnel is near the catalytic triad (S111, H188, D162). Right panel is the same view rotated by 90°. All of the residues that line the tunnel are shown as sticks. The bottleneck, or narrowest point of the tunnel is S111 of the catalytic triad. (B) An electrostatic calculation of the structures shown in panel A. The entrance of the tunnel, which houses the catalytic triad of residues has an overall positive charge, which likely assists in the catalysis. (C) The length of the phosphopantetheine arm of CoA is consistent with the length of the calculated tunnel in ABHD14B. At the exit of the tunnel (yellow) are several candidate residues for interacting with the nucleotide 5’-pyrophosphate moiety of CoA. This view of the tunnel is equivalent to that shown in panel A (right side) and has labels for each of the residues that line the tunnel.